INFORMED CONSENT AN OVERVIEW INTRODUCTION HISTORY DEFINITION ELEMENTS
















































- Slides: 55

INFORMED CONSENT –AN OVERVIEW

INTRODUCTION HISTORY DEFINITION ELEMENTS OF INFORMED CONSENT TYPES OF CONSENT DESIGN LEGAL ASPECTS SUMMARY AND CONCLUSIONS

INTRODUCTION Informed consent is the process of communication between a patient and physician that results in the patient’s authorization or agreement to undergo a specific medical intervention. Both ethical and legal reasons, patients must be given enough information Informed consent, a technical term often used from a legal perspective

The element of consent is one of the critical issues in Research and medical treatment today. It is known that the patient must give valid consent to medical treatment and it is his prerogative to refuse treatment even if the said treatment will save his or her life. No doubt this raises many ethical debates and falls at the heart of medical law today.

HISTORY The earliest expression of this fundamental principle, based on autonomy, is found in the Nuremberg Code of 1947. Declaration of Helsinki emphasizes the importance of obtaining freely given informed consent for medical research

DEFINITION CONSENT in medical practice means an “agreement to accept the consequences of the medical assistance by a patient given to him by a medical practitioner. Two persons are said to be in consent when they agree upon the same thing in the same sense” (section 13 of Indian Contract Act) TREATMENT RESEARCH TRIAL INFORMED CONSENT

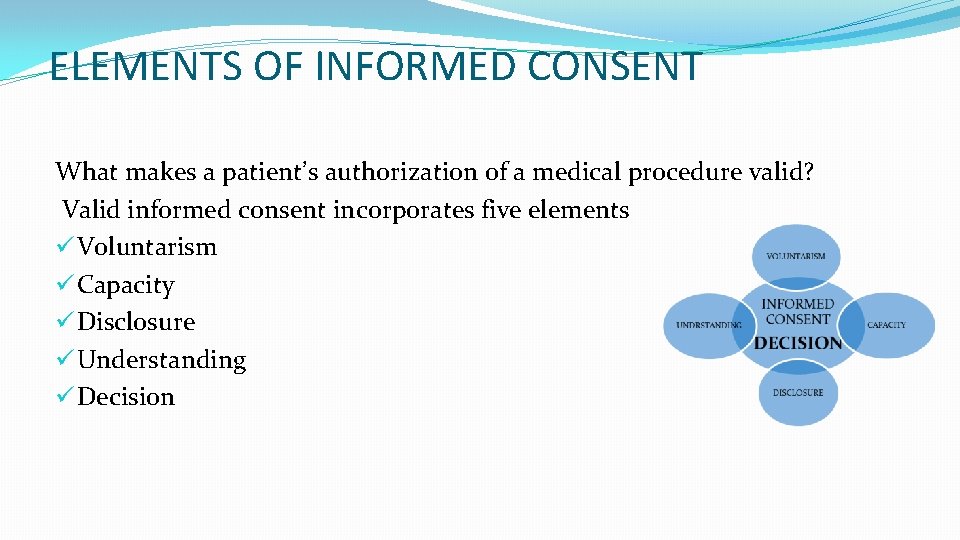
ELEMENTS OF INFORMED CONSENT What makes a patient’s authorization of a medical procedure valid? Valid informed consent incorporates five elements ü Voluntarism ü Capacity ü Disclosure ü Understanding ü Decision

• Voluntarism requires that the patient be free from “coercion and from unfair persuasions and inducements”. Coercion refers to morally inappropriate pressures from individuals or institutions that constrain patients’ exercises of choice

Capacity can be defined as the patient’s ability to make health care decisions. Several criteria can be used clinically to assess a person’s capacity. Competence, a related notion, refers to the patient’s legal standing to make health care decisions. For example, a 16 -year-old may have the capacity to make decisions for him- or herself, but not be competent from a legal point of view.

Disclosure involves providing the patient with the information needed to understand a procedure. This information includes the nature and purpose of the treatment, as well as its risks, potential benefits, and available alternatives. Information should be disclosed using simple explanations In absence of specific statutory or regulatory requirements, the sufficiency of the information provided to the patient is often measured by what other similar practitioners in the community would have disclosed or discussed.

There is little consensus in either law or ethics about what constitutes sufficient understanding. The courts have not generally held that failure of understanding invalidates informed consent. Instead, they have relied on evidence of disclosure when determining if a patient was adequately informed

Decision refers to the patient’s authorization allowing a physician to execute the proposed treatment. Consent forms facilitate and document this authorization but should be secondary to the process through which the patient and the physician discuss and negotiate the proposed treatment.

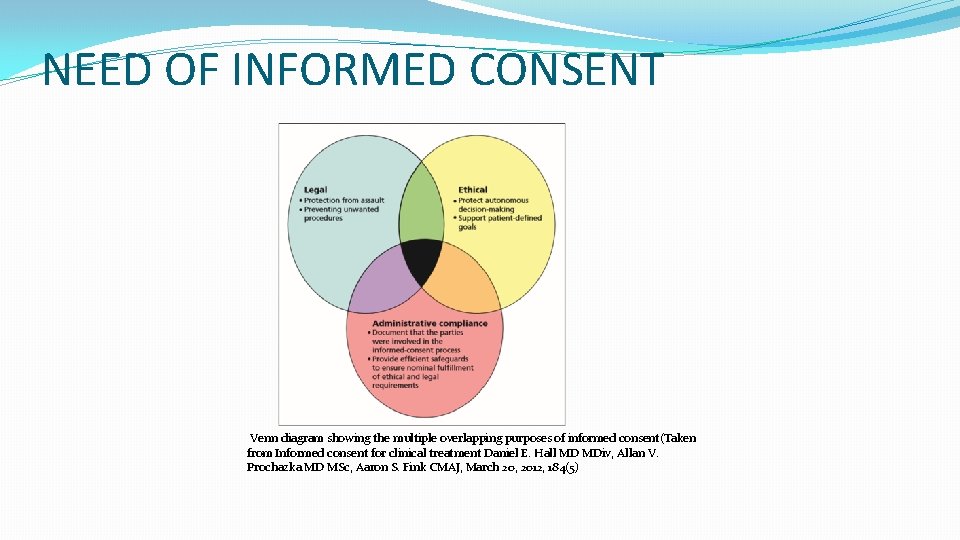
NEED OF INFORMED CONSENT Venn diagram showing the multiple overlapping purposes of informed consent(Taken from Informed consent for clinical treatment Daniel E. Hall MD MDiv, Allan V. Prochazka MD MSc, Aaron S. Fink CMAJ, March 20, 2012, 184(5)

TYPES OF CONSENT Implied Consent. Ø Most commonly variety seen in physician’s practice. Ø There is no written consent but the fact that the patient all by himself comes to the Medical Practitioner shows that he is agreeable to get examined

Express Consent Ø Anything other than implied consent is express consent Ø This can be oral or written.

Proxy Consent Ø These are consents, of any of the above nature given not by the person concerned but by somebody else. Example parent for child, close relative for eventually unsound/unconscious patient, consent given by loco parentis, etc.

DESIGN A well-designed informed consent process would include A description of the proposed surgery, including the anaesthesia to be used Indications for the proposed procedure Risks and benefits for the patient related to the procedure Based on the available clinical evidence Material risks could include risks with a high degree of likelihood but a low degree of severity, as well as those with a very low degree of likelihood but high degree of severity

Treatment alternatives The probable consequences of declining recommended or alternative therapies Who will conduct the procedure Whether physicians other than the operating practitioner, including but not limited to residents, will be performing important tasks related to the surgery, in accordance with the hospital’s policies

An informed consent form in research must include the following: 1. Statementioning that it is research 2. Purpose and methods of the research in simple language 3. Expected duration of the participation and frequency of contact with estimated number of participants to be enrolled, types of data collection and methods 4. Benefits to the participant, community others that might reasonably be expected as an outcome of research 5. Any foreseeable risks, discomfort or inconvenience to the participant resulting from participation in the study

6 Extent to which confidentiality of records could be maintained, such as the limits to which the researcher would be able to safeguard confidentiality and the anticipated consequences of breach of confidentiality 7. Payment/reimbursement for participation and incidental expenses depending on the type of study 8. Free treatment and/or compensation of nparticipants for researchrelated injury and/ or harm

9 Freedom of the individual to participate and/or withdraw from research at any time without penalty or loss of benefits to which the participant would otherwise be entitled 10. The identity of the research team and contact persons with addresses and phone numbers (for example, PI/Co PI for queries related to the research and Chairperson/Member Secretary/ or helpline for appeal against violations of ethical principles and human rights)

11 post research plan/benefit sharing, if research on biological material and/or data leads to commercialization. Publication plan, if any, including photographs and pedigree charts

Adequate time should be given to the participant to read the consent form, if necessar discuss it with family and friends, and seek clarification of her/his doubts from the researchers/research team before deciding to enroll in the research.

Informed consent in children In research involving children, the traditional method of informed consent where decisions about research participation are made by those with the legal and intellectual capacity to make such choices for themselves cannot be implemented, The authority to allow a child’s participation in research rests with parents or a legally acceptable/ authorized representative (LAR), as the case may be. A LAR is an individual or judicial or other body authorized under applicable law to consent on behalf of a prospective participant to participate in research or to undergo a diagnostic, therapeutic, or preventive procedure as per research protocol.

However, investigators must seek to involve children in discussions about research and obtain their assent to participation as in accordance with their developmental level and decision making capacity. The parental/LARs’ permission for the child’s participation in the research is termed as ‘consent’, whereas the child’s agreement to participate is termed as ‘assent’.

There is no need to document assent for children below 7 years of age. For children between 7 and 12 years, verbal/oral assent must be obtained in the presence of the parents/LAR and should be recorded. For children between 12 and 18 years, written assent must be obtained. This assent form also has to be signed by the parents/LAR.

Informed consent in children The EC should determine if consent of one or both parents would be required before a child could be enrolled. Generally, consent from one parent/LAR may be considered sufficient for research involving no more than minimal risk and/or that offers direct benefit to the child. Consent from both parents may have to be obtained when the research involves more than minimal risk and/or offers no benefit to the child.

Classification of Risks Less than minimal risk Probability of harm or discomfort anticipated in the research is nil or not expected. For example, research on anonymous or non-identified data/samples, data available in the public domain, meta-analysis, etc

Minimal risk is defined as those which may be anticipated as harm or discomfort not greater than those ordinarily encountered in daily life This includes procedures such as questioning, observing, and measuring the anthropometric parameters Procedures with minimal risk include history taking, physical examination, chest X-ray, obtaining bodily fluids without invasive intervention, for example, taking saliva or urine samples, etc.

Minor increase over minimal risk or Low risk is defined as a slight increase in the potential for harm or discomfort beyond or more than minimal risk These include procedures that might cause no more than transient pain or tenderness, small bruises or scars, or very slight, temporary distress, such as a blood test, oral sedation for diagnostic procedures, etc. More than minimal risk or High risk All research procedures which have a risk over and above low risk are classified as high risk. These include procedures such as lumbar puncture, lung or liver biopsy, intravenous sedation for diagnostic procedures, etc.

Only one parent’s consent is acceptable if the other parent is deceased, unknown, incompetent, not reasonably available, or when only one parent has legal responsibility for the care and custody of the child, irrespective of the risk involved.

Content of the assent form has to be in accordance with the developmental level and maturity of the children to be enrolled and explained while considering the differences in individual understanding. The language of the assent form must be consistent with the cognitive, social and emotional status of the child. It must be simple and appropriate to the age of the child

The EC may grant consent waiver in the following situations: Research cannot practically be carried out without the waiver and the waiver is scientifically justified Retrospective studies, where the participants are de-identified or cannot be contacted; Research on anonymized biological samples/data Certain types of public health studies/surveillance programmes/programme evaluation studies; Research on data available in the public domain

INFORMED CONSENT TEMPLATE

The ICD has two parts – patient/participant information sheet (PIS) and the informed consent form (ICF). Information on known facts about the research, which has relevance to participation, is included in the PIS. This is followed by the ICF in which the participant acknowledges that she/he has understood the information given in the PIS and is volunteering to be included in that research.



Informed Consent for Clinical Studies Consent for Storage and Future Use of Unused Samples Informed Consent for Qualitative Studies Informed Assent for Children/Minors Informed Parental Consent for Research Involving Children (qualitative) Informed Parental Consent for Research Involving Children (clinical)

In this template: square brackets indicate where specific information is to be inserted Bold lettering indicates sections or wording which should be included Standard lettering is used for explanations to researchers only and must not be included in your consent forms. The explanation is provided in black, and examples are provided in red in italics. Suggested questions to elucidate understanding are given in black in italics.

LEGAL ASPECTS The subsequent discussions will be on the various legal aspects of the specifics of consent taken from different judgements in India There are relatively few reported cases at the level of the Supreme Court on the question of obtaining a patient’s consent. The Supreme Court’s decision in Samira Kohli vs Dr D Manchanda provides useful guidance to medical professionals. This judgement acted as a basis for many of the future decisions on consent

The honorable court mentioned Ø Where a patient’s consent is taken for a diagnostic procedure or surgery, such consent cannot be categorised as permission to perform therapeutic surgery, whether conservative or radical, except in life threatening situations. Ø Where a patient’s consent is taken for a particular procedure, that consent cannot be used for an additional procedure

In this judgment the judge also mentioned about difference in the Real and informed consent Real consent as followed in U K and informed consent as followed in U S and then decided that the UK definition should be followed in the Indian context

The following excerpt from the judgment provides why the consent as adopted in U K is better to our Indian context than the U S Laws There is a need to keep the cost of treatment within affordable limits. Bringing in the American concepts and standards of treatment procedures and disclosure of risks, consequences and choices will inevitably bring in the higher cost structure of American medical care. Patients in India cannot afford them

Explaining what is ‘adequate information’ to be furnished by the doctor court said ‘Doctor who treats the patient, should enable the patient to make a balanced judgment as to whether he should submit himself to the particular treatment or not. there is no need to explain the remote or theoretical risks involved, which may frighten or confuse a patient and result in refusal of consent for the necessary treatment.

Similarly, there is no need to explain the remote or theoretical risks of refusal to take treatment which may persuade a patient to undergo a fanciful or unnecessary treatment. A balance should be achieved between the need for disclosing necessary and adequate information and at the same time avoid the possibility of the patient being deterred from agreeing to a necessary treatment or offering to undergo an unnecessary treatment’.

DO S AND DON’TS IN INFORMED CONSENT (summary of various other judgments) Never leave consent form incomplete as Incomplete or incorrectly filled consent form could be construed as negligence The doctor signing the consent form must specifically write down his name on the consent form. Do not take “blanket” consent from the patient without giving relevant information as blanket consent is not a valid consent Always take consent after explaining the patient about the proposed treatment / associated risks / alternatives and do not ask the patient to sign on ‘dotted lines’

It is advisable that the consent form should be filled by one person, in one ink, and in one sitting. Attestation by a witness on the consent or taking a simple declaration is always advisable. Do take a Specific and separate consent to operate during workshop/ conference/ seminar

CONSENT IN EMERGENCY It is an important exception to the rule. In cases of emergency a patient may be unable to give consent, in such cases a substitute decision maker, if readily available, should be approached. If however such a person is not on the scene, then it is the duty of the Doctor to do what is essential to save life even without consent. For the doctor to declare any clinical situation an emergency, for which consent is not required there should be demonstrable imminent threat to the life or health of the patient.

There must be an undoubted necessity to proceed at that time. Under such emergency situations, the treatment should be limited to those steps which are necessary to deal with, imminent threat to life, limb or health. If the circumstances are such that the urgency might be questioned later, arranging a second medical opinion would be prudent, if it is possible to do so. (Taken from http: //www. apiindia. org/pdf/medicine_update_2007/153. pdf)

Absence of written consent is not a serious deficiency or lapse on the part of doctor during emergency procedure During emergency no consent of the patient / attendants is required to transfer a hospitalized patient to another hospital, when absolutely indicated

SUMMARY AND CONCLUSIONS Ø Informed consent is the process of communication between a patient and physician Ø It is a continuous process and not to be treated as mere one-time document and get restricted to a piece of paper Ø It helps in maintenance of patient’s autonomy and his active involvement while he is treated upon Ø Ethical dilemma still exists for the clinician when question autonomy vs best treatment for the patient crop up

Ø Because of the confusion and panic on informed consent, doctors are scurrying to prepare various ‘legally fool proof’ consent forms. Ø A signed consent form of any nature neither guarantees a doctor protection against legal action nor ensures patient satisfaction. Ø It merely demonstrates that some process to exchange information was followed.

Ø The debate about informed consent needs to move away from the legal aspect to the domain of ethics where it really belongs. Unless ethical standards in the profession are enforced, medico-legal aspects of informed consent will continue to haunt doctors. Ø Though few thinkers have advocated for allowing beach of some amount of autonomy in certain situations by and large most of the courts do have given more importance to autonomy of the patients

we should be doing a disservice to the community at large if we were to impose liability on hospitals and doctors for everything that happens to go wrong we must not condemn as negligent that which is only misadventure. “ LORD DANNING
