Information technology and informatics enhancement of clinical trial













- Slides: 20

Information technology and informatics enhancement of clinical trial programs Presented by Matthew Seguin, Director of Clinical Knowledge Management Capital Technology Information Services, Inc. Shaw Pittman - Health. Care Technology Network Meeting Topic: Technology for Clinical Trials on November 19, 2004 in Washington, DC CTIS – Capital Technology Information Services, Inc. Proprietary Information

Material that will be covered today l CTIS general background information l Critical parts of a IT enabled Clinical Trial Informatics solution l Integration of lessons learned and industry best practice into a customized working solution l Review of IT enabled ROI in Clinical Trials program performance l Summary l Questions CTIS – Capital Technology Information Services, Inc. Proprietary Information

Capital Technology Information Services, Inc. (CTIS) Background Information CTIS – Capital Technology Information Services, Inc. Proprietary Information

Why was CTIS invited here today? l CTIS provides information technology and informatics support services for the largest Clinical Trial Networks in the US and abroad. l l Cancer Therapy Evaluation Program (CTEP) l Division of AIDS Program (DAIDS) Mr. Seguin is the Director of Clinical Knowledge Management Systems for CTIS. His principal clients are NIH’s DAIDS and CTEP programs. He is responsible for providing clinical trial informatics and clinical trial management subject matter expertise in the development of their enterprise-wide information management systems, decision support strategies, and good clinical practice implementation. CTIS – Capital Technology Information Services, Inc. Proprietary Information

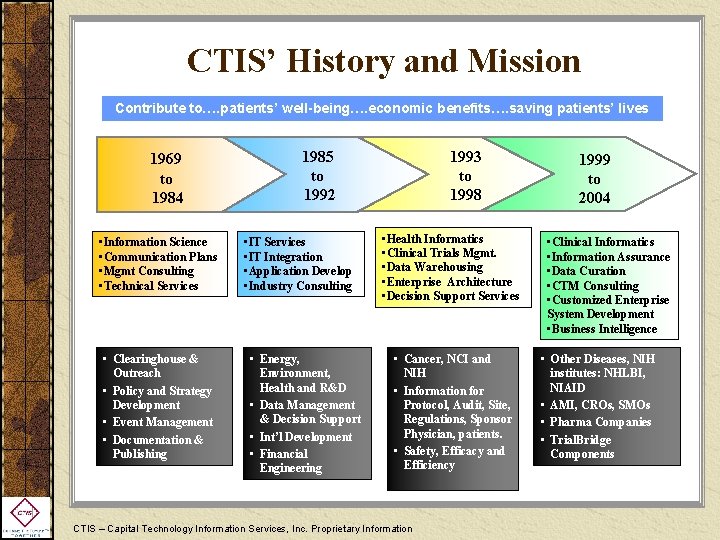
CTIS’ History and Mission Contribute to…. patients’ well-being…. economic benefits…. saving patients’ lives 1969 to 1984 • Information Science • Communication Plans • Mgmt Consulting • Technical Services • Clearinghouse & Outreach • Policy and Strategy Development • Event Management • Documentation & Publishing 1985 to 1992 • IT Services • IT Integration • Application Develop • Industry Consulting • Energy, Environment, Health and R&D • Data Management & Decision Support • Int’l Development • Financial Engineering 1993 to 1998 • Health Informatics • Clinical Trials Mgmt. • Data Warehousing • Enterprise Architecture • Decision Support Services • Cancer, NCI and NIH • Information for Protocol, Audit, Site, Regulations, Sponsor Physician, patients. • Safety, Efficacy and Efficiency CTIS – Capital Technology Information Services, Inc. Proprietary Information 1999 to 2004 • Clinical Informatics • Information Assurance • Data Curation • CTM Consulting • Customized Enterprise System Development • Business Intelligence • Other Diseases, NIH institutes: NHLBI, NIAID • AMI, CROs, SMOs • Pharma Companies • Trial. Bridge Components

Critical parts of a IT enabled Clinical Trial Informatics solution CTIS – Capital Technology Information Services, Inc. Proprietary Information

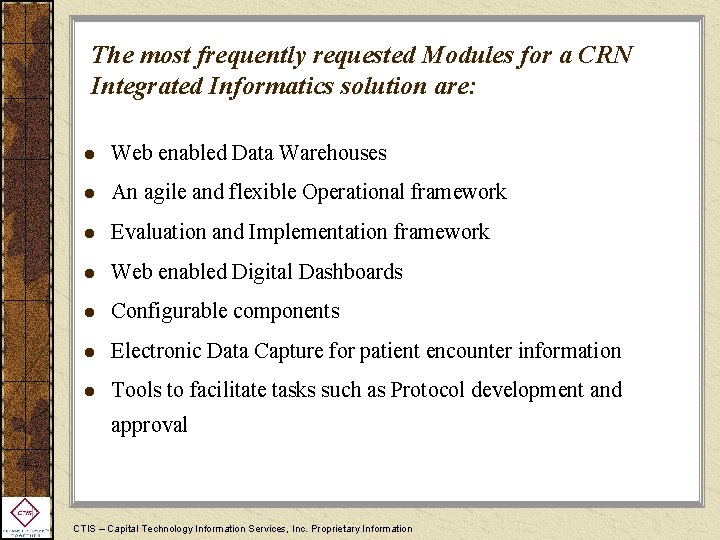
The most frequently requested Modules for a CRN Integrated Informatics solution are: l Web enabled Data Warehouses l An agile and flexible Operational framework l Evaluation and Implementation framework l Web enabled Digital Dashboards l Configurable components l Electronic Data Capture for patient encounter information l Tools to facilitate tasks such as Protocol development and approval CTIS – Capital Technology Information Services, Inc. Proprietary Information

These Clinical Research management Modules need to address key business realities or desires l More INDs and Agents are processed through the CT process l Efficient and effective use of available resources l Open communication practices l Workflow improvement without re-engineering the process l Reduce resource duplication and enhance throughput l Empower the people to manage change l Embed quality measures l Standardized compliance frameworks l Share information across infrastructures l Hospitals and resource poor clinics trial participation CTIS – Capital Technology Information Services, Inc. Proprietary Information

These key business realities or desires are enabled through IT solutions that are highly efficient and effective l Rapid exchange of concepts and protocols l Avoid service fragmentation l Manage strategic direction implementation l Facilitate workflow l Enhance information sharing l Reuse existing infrastructure l Enable oversight, monitoring, and measurement capabilities l Dynamic maintenance of Standardized Operating Procedures l Demonstrate a measurable ROI soon after implementation CTIS – Capital Technology Information Services, Inc. Proprietary Information

These solutions will to map to key CT program objectives and will help drive Informatics solution Many Clinical Trial Informatics driven programs have 12 objectives, which are: l l l Optimization of the clinical research process Building of awareness and outreach Capacity building and training Information flow and management processes Resource allocation and optimization Regulatory compliance Standards management Safety management and reporting Oversight and monitoring framework SOP framework Interface with Support organizations Stable and Standardized IT infrastructure backbone CTIS – Capital Technology Information Services, Inc. Proprietary Information

Integration of lessons learned and industry best practice into a customized working solution National Cancer Institute, Cancer Therapy and Evaluation Program and National Institute of Allergy and Infectious Disease, Division of AIDS Program CTIS – Capital Technology Information Services, Inc. Proprietary Information

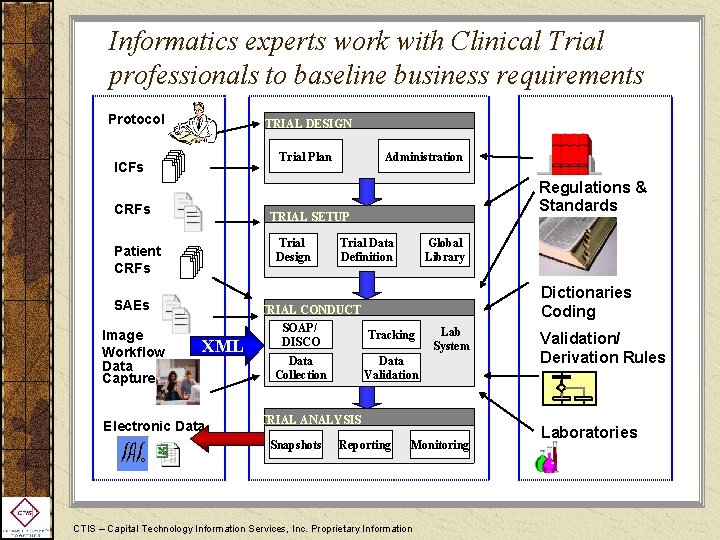
Informatics experts work with Clinical Trial professionals to baseline business requirements Protocol TRIAL DESIGN Trial Plan ICFs CRFs Regulations & Standards TRIAL SETUP Trial Design Patient CRFs SAEs Image Workflow Data Capture Administration XML Electronic Data Trial Data Definition Global Library TRIAL CONDUCT SOAP/ Tracking DISCO Data Collection Dictionaries Coding Lab System Data Validation TRIAL ANALYSIS Snapshots Reporting Monitoring CTIS – Capital Technology Information Services, Inc. Proprietary Information Validation/ Derivation Rules Laboratories

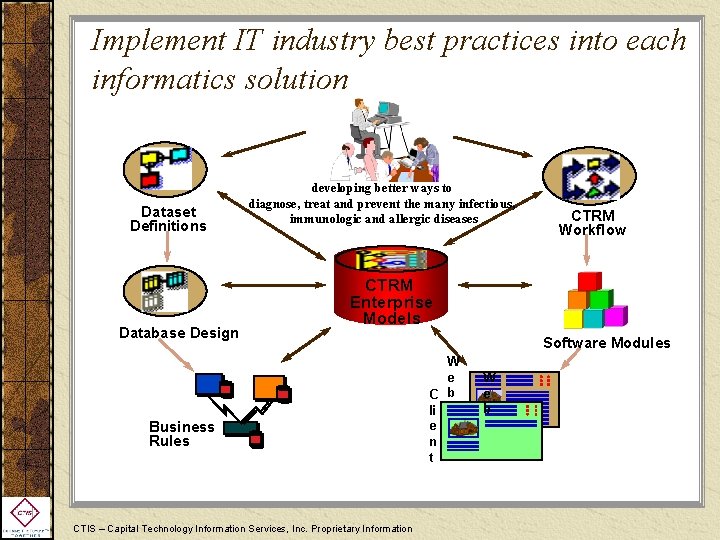
Implement IT industry best practices into each informatics solution Dataset Definitions Database Design developing better ways to diagnose, treat and prevent the many infectious, immunologic and allergic diseases CTRM Workflow CTRM Enterprise Models Business Rules CTIS – Capital Technology Information Services, Inc. Proprietary Information Software Modules W e C b li e n t W e b

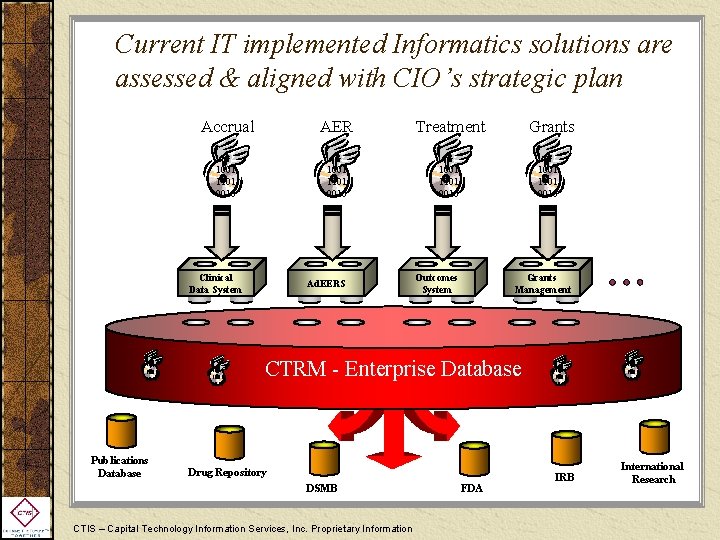
Current IT implemented Informatics solutions are assessed & aligned with CIO’s strategic plan Accrual AER Treatment 1001 1101 0010 Ad. EERS Outcomes System Clinical Data System 1001 1101 0010 Publications Database 1001 1101 0010 Grants Management CTRM - Enterprise Database Drug Repository DSMB CTIS – Capital Technology Information Services, Inc. Proprietary Information FDA 1001 1101 0010 IRB 1001 1101 0010 International Research

Devise Informatics solutions that empower Clinical Trial and Program Management professionals How many grade III or higher SAE's have been reported in the past year, for subjects enrolled in clinical trials of anti -retroviral drugs in the US vs. Sub-Saharan Africa, what were they and how were they distributed by SAE type and across gender and race? EIS Query in English Output in Multiple output formats S Q L Query Analyzer 01101100 CTRM - Enterprise Database Aligned with CIO strategic plan CTIS – Capital Technology Information Services, Inc. Proprietary Information

Review of IT enabled ROI in Clinical Trials Performance CTIS – Capital Technology Information Services, Inc. Proprietary Information

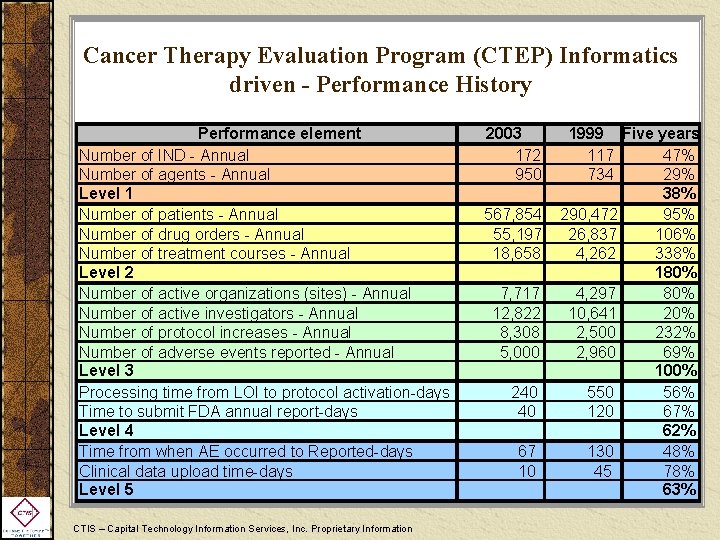
Cancer Therapy Evaluation Program (CTEP) Informatics driven - Performance History Performance element Number of IND - Annual Number of agents - Annual Level 1 Number of patients - Annual Number of drug orders - Annual Number of treatment courses - Annual Level 2 Number of active organizations (sites) - Annual Number of active investigators - Annual Number of protocol increases - Annual Number of adverse events reported - Annual Level 3 Processing time from LOI to protocol activation-days Time to submit FDA annual report-days Level 4 Time from when AE occurred to Reported-days Clinical data upload time-days Level 5 CTIS – Capital Technology Information Services, Inc. Proprietary Information 2003 172 950 567, 854 55, 197 18, 658 7, 717 12, 822 8, 308 5, 000 240 40 67 10 1999 Five years 117 47% 734 29% 38% 290, 472 95% 26, 837 106% 4, 262 338% 180% 4, 297 80% 10, 641 20% 2, 500 232% 2, 960 69% 100% 550 56% 120 67% 62% 130 48% 45 78% 63%

Summary l Customized IT CT systems can help professionals manage change l Identification business process restrictions opens up IT options l Work in partnership to target problem areas and devise solutions l Align current systems with CIO strategic plan l Implement proven & applicable Industry Best Practice solutions l Link business process vital signs to Digital Dashboards l Implement modular configurable components l Create and standardize interoperable enterprise IT architecture l Guarantee data accuracy throughout a secure chain of custody CTIS – Capital Technology Information Services, Inc. Proprietary Information

CTIS Contact Information l Matthew Seguin l Chris Stathes Director of Clinical Knowledge Executive Vice President Management One Research Court Suite 200 Rockville, MD 20850 Office: (301) 948 -3033 CTIS – Capital Technology Information Services, Inc. Proprietary Information

Questions CTIS – Capital Technology Information Services, Inc. Proprietary Information
 Image enhancement in night vision technology
Image enhancement in night vision technology Cynchia
Cynchia Rsna ctp anonymizer
Rsna ctp anonymizer Morpheus bms
Morpheus bms Clinical trial budget example
Clinical trial budget example Novel clinical drug trial design
Novel clinical drug trial design Clinicaltrials.gov api
Clinicaltrials.gov api Clinical trial financial management
Clinical trial financial management Phase 4 trial
Phase 4 trial Nida clinical trial network
Nida clinical trial network Ivd clinical trial design
Ivd clinical trial design Clinical trial exports
Clinical trial exports Clinical trial worksheet
Clinical trial worksheet Master clinical trial agreement
Master clinical trial agreement Clinical trial matching service
Clinical trial matching service Ui division of sponsored programs
Ui division of sponsored programs Trofinetide rett
Trofinetide rett Accelerated clinical trial agreement acta
Accelerated clinical trial agreement acta Mosaico clinical trial
Mosaico clinical trial Prs clinical trial
Prs clinical trial Clinical trial centers alliance
Clinical trial centers alliance