Information given by chemical equations 2 C 6
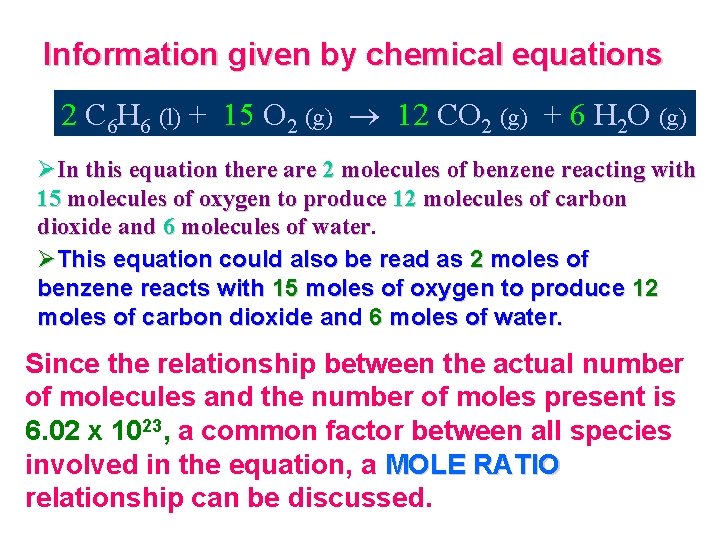
Information given by chemical equations 2 C 6 H 6 (l) + 15 O 2 (g) 12 CO 2 (g) + 6 H 2 O (g) ØIn this equation there are 2 molecules of benzene reacting with 15 molecules of oxygen to produce 12 molecules of carbon dioxide and 6 molecules of water ØThis equation could also be read as 2 moles of benzene reacts with 15 moles of oxygen to produce 12 moles of carbon dioxide and 6 moles of water. Since the relationship between the actual number of molecules and the number of moles present is 6. 02 x 1023, a common factor between all species involved in the equation, a MOLE RATIO relationship can be discussed.
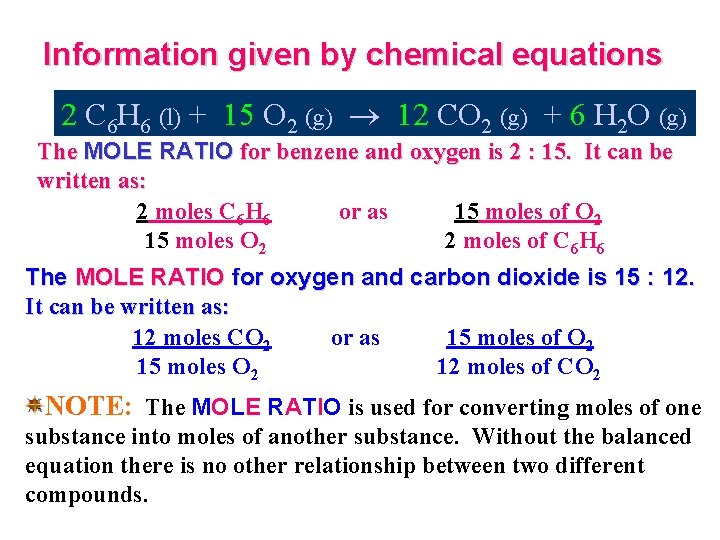
Information given by chemical equations 2 C 6 H 6 (l) + 15 O 2 (g) 12 CO 2 (g) + 6 H 2 O (g) The MOLE RATIO for benzene and oxygen is 2 : 15. It can be written as: 2 moles C 6 H 6 or as 15 moles of O 2 15 moles O 2 2 moles of C 6 H 6 The MOLE RATIO for oxygen and carbon dioxide is 15 : 12. It can be written as: 12 moles CO 2 or as 15 moles of O 2 15 moles O 2 12 moles of CO 2 NOTE: The MOLE RATIO is used for converting moles of one substance into moles of another substance. Without the balanced equation there is no other relationship between two different compounds.
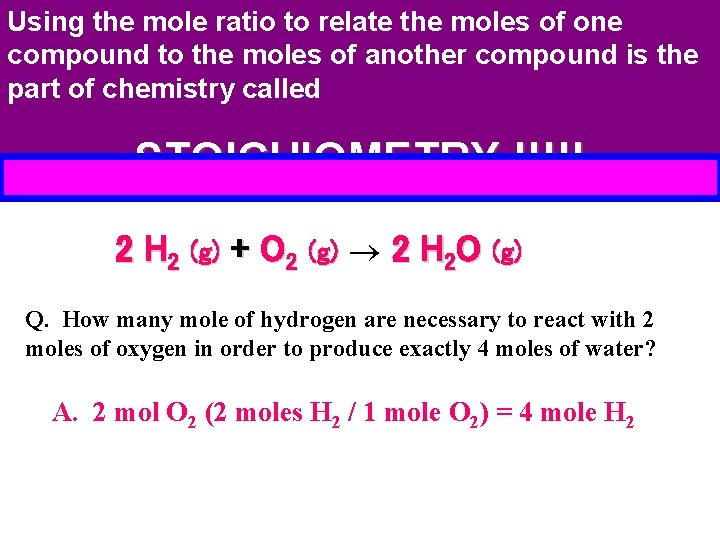
Using the mole ratio to relate the moles of one compound to the moles of another compound is the part of chemistry called STOICHIOMETRY !!!!! 2 H 2 (g) + O 2 (g) 2 H 2 O (g) Q. How many mole of hydrogen are necessary to react with 2 moles of oxygen in order to produce exactly 4 moles of water? A. 2 mol O 2 (2 moles H 2 / 1 mole O 2) = 4 mole H 2
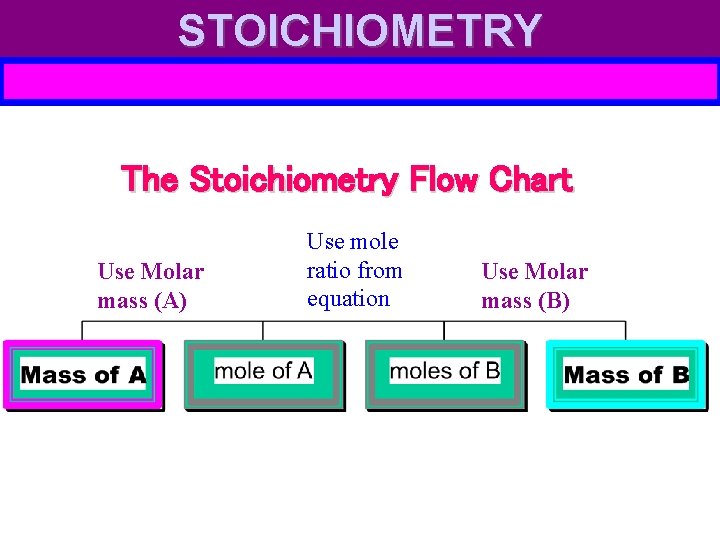
STOICHIOMETRY The Stoichiometry Flow Chart Use Molar mass (A) Use mole ratio from equation Use Molar mass (B)
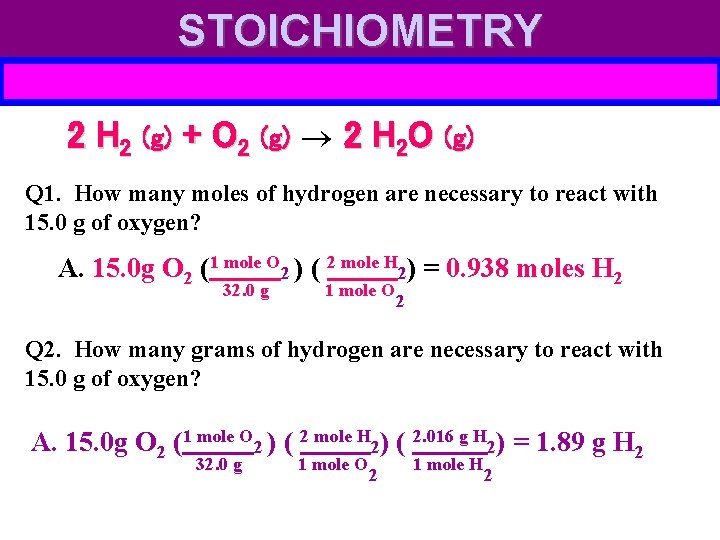
STOICHIOMETRY 2 H 2 (g) + O 2 (g) 2 H 2 O (g) Q 1. How many moles of hydrogen are necessary to react with 15. 0 g of oxygen? A. 15. 0 g O 2 (1 mole O 2 ) ( 2 mole H 2) = 0. 938 moles H 2 32. 0 g 1 mole O 2 Q 2. How many grams of hydrogen are necessary to react with 15. 0 g of oxygen? A. 15. 0 g O 2 (1 mole O 2 ) ( 2 mole H 2) ( 2. 016 g H 2) = 1. 89 g H 2 32. 0 g 1 mole O 2 1 mole H 2
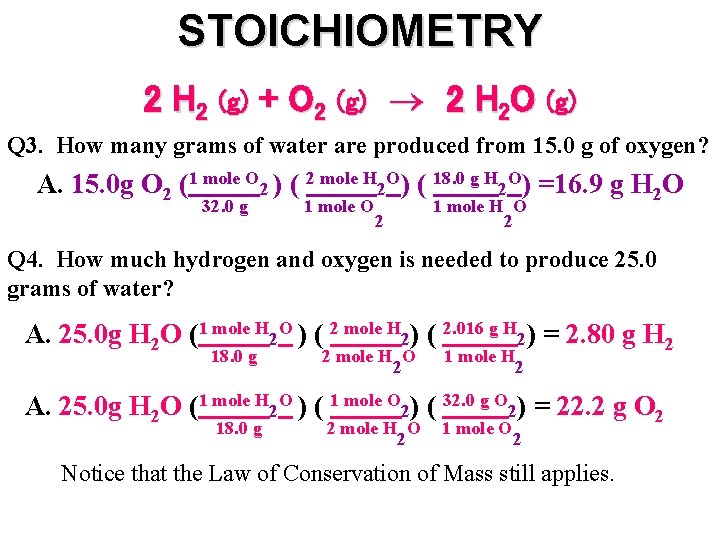
STOICHIOMETRY 2 H 2 (g) + O 2 (g) 2 H 2 O (g) Q 3. How many grams of water are produced from 15. 0 g of oxygen? A. 15. 0 g O 2 (1 mole O 2 ) ( 2 mole H 2 O) ( 18. 0 g H 2 O) =16. 9 g H 2 O 32. 0 g 1 mole O 2 1 mole H O 2 Q 4. How much hydrogen and oxygen is needed to produce 25. 0 grams of water? A. 25. 0 g H 2 O (1 mole H 2 O ) ( 2 mole H 2) ( 2. 016 g H 2) = 2. 80 g H 2 18. 0 g 2 mole H O 2 1 mole H 2 A. 25. 0 g H 2 O (1 mole H 2 O ) ( 1 mole O 2) ( 32. 0 g O 2) = 22. 2 g O 2 18. 0 g 2 mole H O 2 1 mole O 2 Notice that the Law of Conservation of Mass still applies.
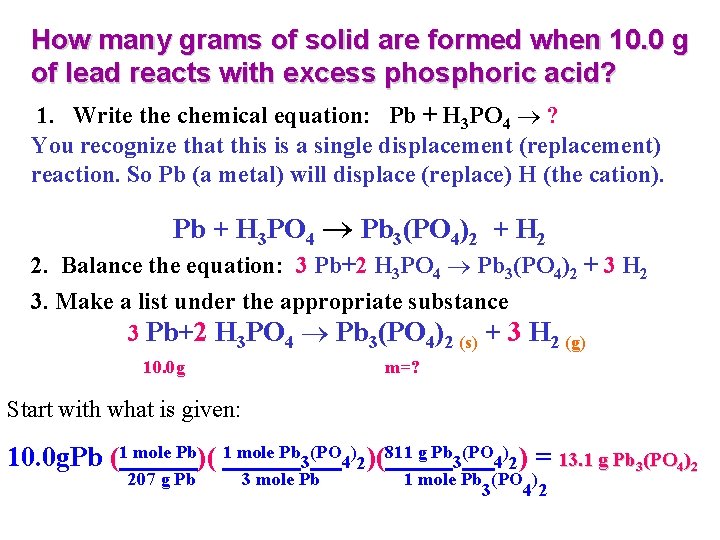
How many grams of solid are formed when 10. 0 g of lead reacts with excess phosphoric acid? 1. Write the chemical equation: Pb + H 3 PO 4 ? You recognize that this is a single displacement (replacement) reaction. So Pb (a metal) will displace (replace) H (the cation). Pb + H 3 PO 4 Pb 3(PO 4)2 + H 2 2. Balance the equation: 3 Pb+2 H 3 PO 4 Pb 3(PO 4)2 + 3 H 2 3. Make a list under the appropriate substance 3 Pb+2 H 3 PO 4 Pb 3(PO 4)2 (s) + 3 H 2 (g) 10. 0 g m=? Start with what is given: 10. 0 g. Pb (1 mole Pb)( 1 mole Pb 3(PO 4)2)(811 g Pb 3(PO 4)2) = 13. 1 g Pb 3(PO 4)2 207 g Pb 3 mole Pb 1 mole Pb (PO ) 3 42
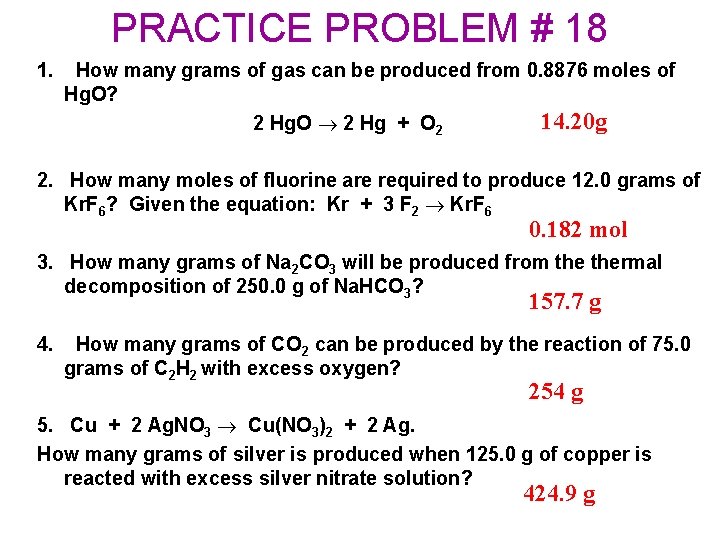
PRACTICE PROBLEM # 18 1. How many grams of gas can be produced from 0. 8876 moles of Hg. O? 14. 20 g 2 Hg. O 2 Hg + O 2 2. How many moles of fluorine are required to produce 12. 0 grams of Kr. F 6? Given the equation: Kr + 3 F 2 Kr. F 6 0. 182 mol 3. How many grams of Na 2 CO 3 will be produced from thermal decomposition of 250. 0 g of Na. HCO 3? 157. 7 g 4. How many grams of CO 2 can be produced by the reaction of 75. 0 grams of C 2 H 2 with excess oxygen? 254 g 5. Cu + 2 Ag. NO 3 Cu(NO 3)2 + 2 Ag. How many grams of silver is produced when 125. 0 g of copper is reacted with excess silver nitrate solution? 424. 9 g
GROUP STUDY PROBLEM # 18 ______1. How many grams of liquid product can be produced from 3. 55 moles of Hg. O? 2 Hg. O 2 Hg + O 2 ______2. How many moles of fluorine are required to produce 3. 0 grams of Kr. F 6? Given the equation: Kr + 3 F 2 Kr. F 6 ______3. How many grams of Na 2 CO 3 will be produced from the decomposition of 20. 0 g of Na. HCO 3? ______4. C 2 H 4 ? How many grams of O 2 are needed to combust 55. 0 grams of ______5. Cu + 2 Ag. NO 3 Cu(NO 3)2 + 2 Ag. How many grams of silver is produced when 50. 0 g of copper is reacted with excess silver nitrate solution?
- Slides: 9