Information and Thermodynamic Entropy or Waiting for Landauer






















- Slides: 41

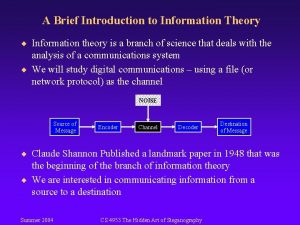
Information and Thermodynamic Entropy or, Waiting for Landauer John D. Norton Department of History and Philosophy of Science Center for Philosophy of Science University of Pittsburgh CARL FRIEDRICH VON WEIZSÄCKER LECTURES UNIVERSITY OF HAMBURG June 2010 1

Philosophy and Physics Information ideas and concepts = Entropy heat, work, thermodynamics And why not? Mass = Energy Particles = Waves Geometry = Gravity …. Time = Money 2

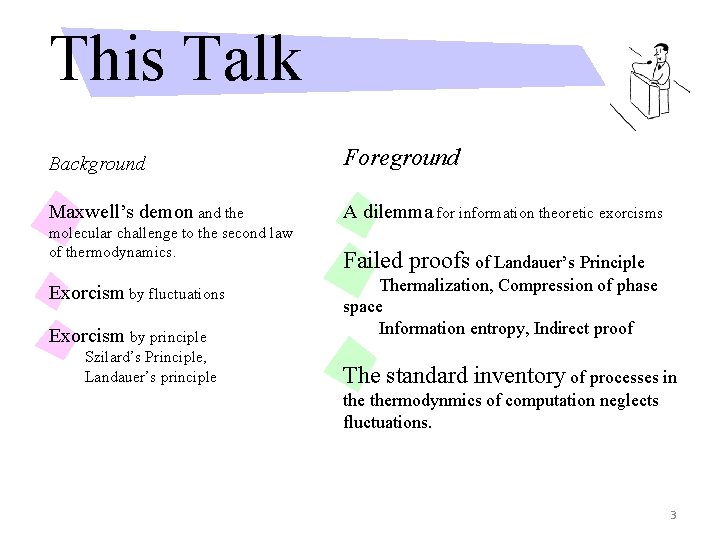
This Talk Background Foreground Maxwell’s demon and the A dilemma for information theoretic exorcisms molecular challenge to the second law of thermodynamics. Exorcism by fluctuations Exorcism by principle Szilard’s Principle, Landauer’s principle Failed proofs of Landauer’s Principle Thermalization, Compression of phase space Information entropy, Indirect proof The standard inventory of processes in thermodynmics of computation neglects fluctuations. 3

Maxwell’s demon 4

The original conception Demon operates door intelligently Divided chamber with a kinetic gas. J. C. Maxwell in a letter to P. G. Tait, 11 th December 1867 “…the hot system has got hotter and the cold system colder and yet no work has been done, only the intelligence of a very observant and neatfingered being has been employed. ” “[T]he 2 nd law of thermodynamics has the same degree of truth as the statement that if you throw a tumblerful of water into the sea you cannot get the same tumblerful of water out again. ” 5

The Devil lives in the details of Brownian motion and other fluctuations “…we see under out eyes now motion transformed into heat by friction, now heat changed inversely into motion, and that without loss since the movement lasts forever. That is the contrary of the principle of Carnot. ” Poincaré, 1907 Could these momentary, miniature violations of the second law be accumulated to large-scale violations? Guoy (1888), Svedberg (1907) designed minimachines with that purpose. 6

Exorcism by Fluctuation 7

Marian Smoluchowski, 1912 The best known of many examples. Trapdoor hinged so that fast molecules moving from left to right swing it open and pass, but not vice versa. BUT AND SO The trapdoor must be very light so a molecule can swing it open. The trapdoor has its own thermal energy of k. T/2 per degree of freedom. The trapdoor will flap about wildly and let molecules pass in both directions. The second law holds on average only over time. Machines that try to accumulate fluctuations are disrupted fatally by them. 8

Szilard’s One-Molecule Engine 9

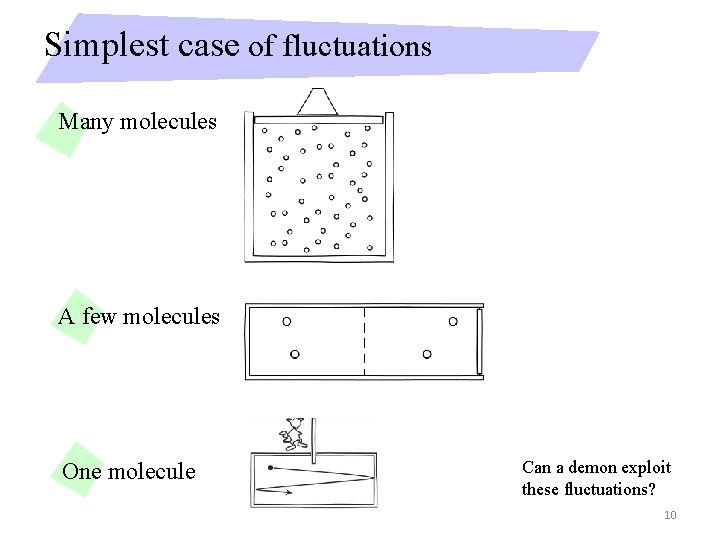
Simplest case of fluctuations Many molecules A few molecules One molecule Can a demon exploit these fluctuations? 10

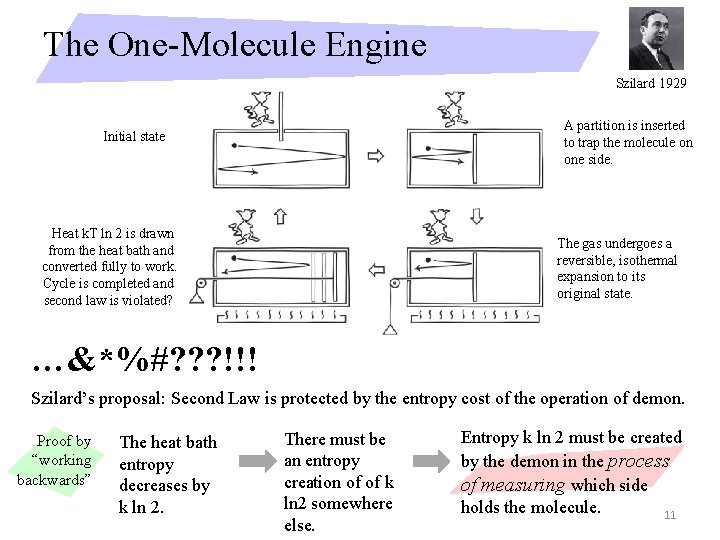
The One-Molecule Engine Szilard 1929 A partition is inserted to trap the molecule on one side. Initial state Heat k. T ln 2 is drawn from the heat bath and converted fully to work. Cycle is completed and second law is violated? The gas undergoes a reversible, isothermal expansion to its original state. …&*%#? ? ? !!! Szilard’s proposal: Second Law is protected by the entropy cost of the operation of demon. Proof by “working backwards” The heat bath entropy decreases by k ln 2. There must be an entropy creation of of k ln 2 somewhere else. Entropy k ln 2 must be created by the demon in the process of measuring which side holds the molecule. 11

Exorcism by principle 12

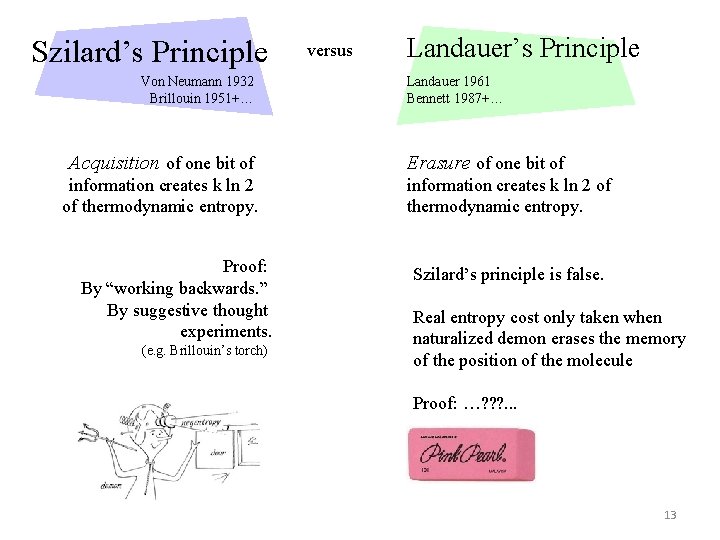
Szilard’s Principle Von Neumann 1932 Brillouin 1951+… Acquisition of one bit of information creates k ln 2 of thermodynamic entropy. Proof: By “working backwards. ” By suggestive thought experiments. (e. g. Brillouin’s torch) versus Landauer’s Principle Landauer 1961 Bennett 1987+… Erasure of one bit of information creates k ln 2 of thermodynamic entropy. Szilard’s principle is false. Real entropy cost only taken when naturalized demon erases the memory of the position of the molecule Proof: …? ? ? . . . 13

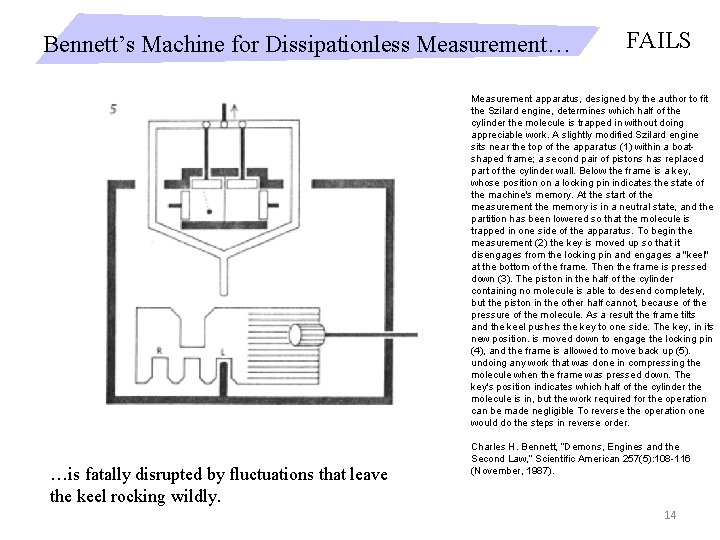
Bennett’s Machine for Dissipationless Measurement… FAILS Measurement apparatus, designed by the author to fit the Szilard engine, determines which half of the cylinder the molecule is trapped in without doing appreciable work. A slightly modified Szilard engine sits near the top of the apparatus (1) within a boatshaped frame; a second pair of pistons has replaced part of the cylinder wall. Below the frame is a key, whose position on a locking pin indicates the state of the machine's memory. At the start of the measurement the memory is in a neutral state, and the partition has been lowered so that the molecule is trapped in one side of the apparatus. To begin the measurement (2) the key is moved up so that it disengages from the locking pin and engages a "keel" at the bottom of the frame. Then the frame is pressed down (3). The piston in the half of the cylinder containing no molecule is able to desend completely, but the piston in the other half cannot, because of the pressure of the molecule. As a result the frame tilts and the keel pushes the key to one side. The key, in its new position. is moved down to engage the locking pin (4), and the frame is allowed to move back up (5). undoing any work that was done in compressing the molecule when the frame was pressed down. The key's position indicates which half of the cylinder the molecule is in, but the work required for the operation can be made negligible To reverse the operation one would do the steps in reverse order. …is fatally disrupted by fluctuations that leave the keel rocking wildly. Charles H. Bennett, “Demons, Engines and the Second Law, ” Scientific American 257(5): 108 -116 (November, 1987). 14

A dilemma for information theoretic exorcisms 15

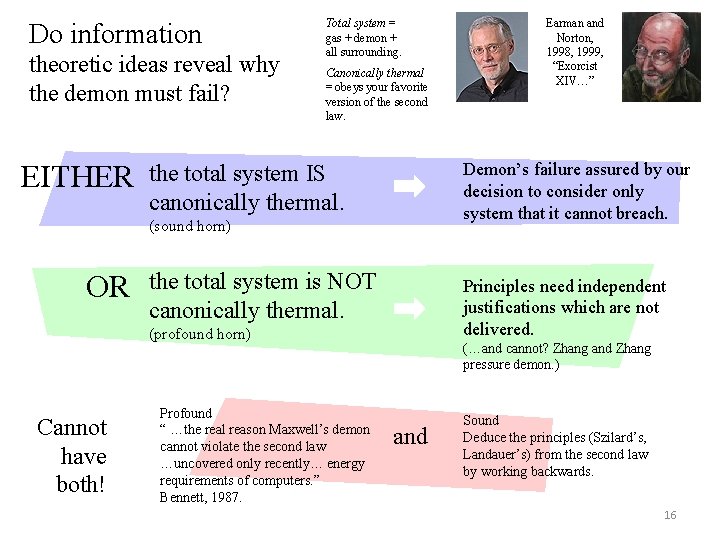
Do information theoretic ideas reveal why the demon must fail? EITHER Total system = gas + demon + all surrounding. Canonically thermal = obeys your favorite version of the second law. Demon’s failure assured by our decision to consider only system that it cannot breach. the total system IS canonically thermal. (sound horn) OR the total system is NOT canonically thermal. Principles need independent justifications which are not delivered. (profound horn) Cannot have both! Profound “ …the real reason Maxwell’s demon cannot violate the second law …uncovered only recently… energy requirements of computers. ” Bennett, 1987. Earman and Norton, 1998, 1999, “Exorcist XIV…” (…and cannot? Zhang and Zhang pressure demon. ) and Sound Deduce the principles (Szilard’s, Landauer’s) from the second law by working backwards. 16

Failed proofs of Landauer’s Principle 17

1. 18

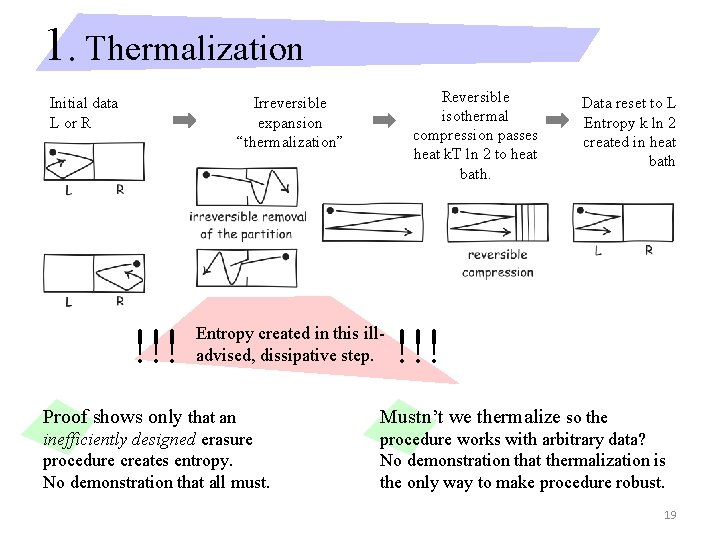
1. Thermalization Reversible isothermal compression passes heat k. T ln 2 to heat bath. Irreversible expansion “thermalization” Initial data L or R !!! Entropy created in this illadvised, dissipative step. Data reset to L Entropy k ln 2 created in heat bath !!! Proof shows only that an Mustn’t we thermalize so the inefficiently designed erasure procedure creates entropy. No demonstration that all must. procedure works with arbitrary data? No demonstration that thermalization is the only way to make procedure robust. 19

2. 20

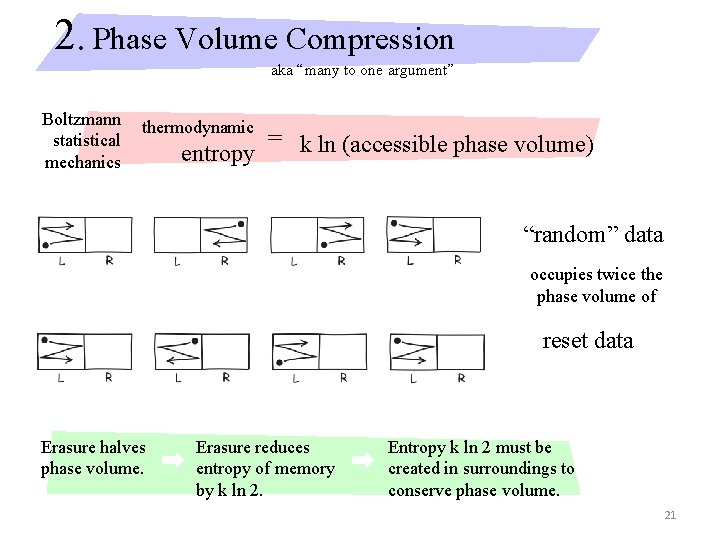
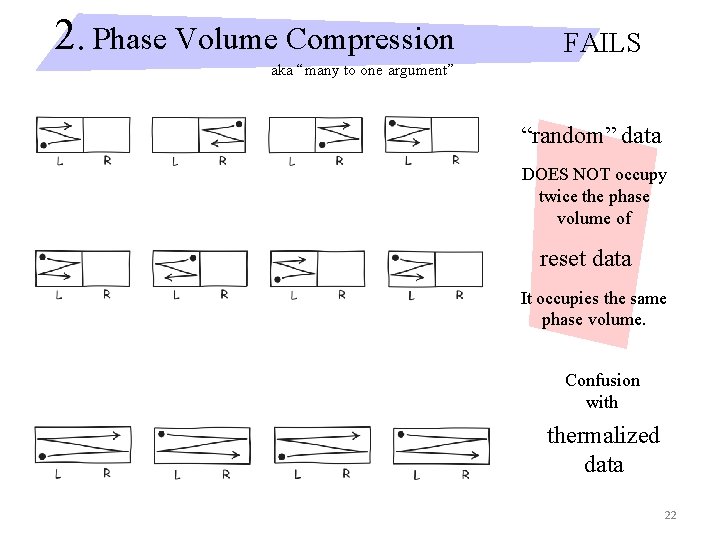
2. Phase Volume Compression aka “many to one argument” Boltzmann statistical mechanics thermodynamic entropy = k ln (accessible phase volume) “random” data occupies twice the phase volume of reset data Erasure halves phase volume. Erasure reduces entropy of memory by k ln 2. Entropy k ln 2 must be created in surroundings to conserve phase volume. 21

2. Phase Volume Compression FAILS aka “many to one argument” “random” data DOES NOT occupy twice the phase volume of reset data It occupies the same phase volume. Confusion with thermalized data 22

A Ruinous Sense of “Reversible” Random data and insertion of the partition removal of the partition thermalized data have the same entropy because they are connected by a reversible, adiabatic process? ? ? DS = 0 random data thermalized data No. Under this sense of reversible, entropy ceases to be a state function. DS = k ln 2 23

3. 24

3. Information-theoretic Entropy “p ln p” Information entropy S = - k Si inf Pi ln Pi “random” data PL = PR = 1/2 Sinf = k ln 2 reset data PL = 1; PR = 0 Sinf = 0 Hence erasure reduces the entropy of the memory by k ln 2, which must appear in surroundings. But… in this case, Information entropy does NOT equal Thermodynamic entropy is attached to a probability only in special cases. Not this one. 25

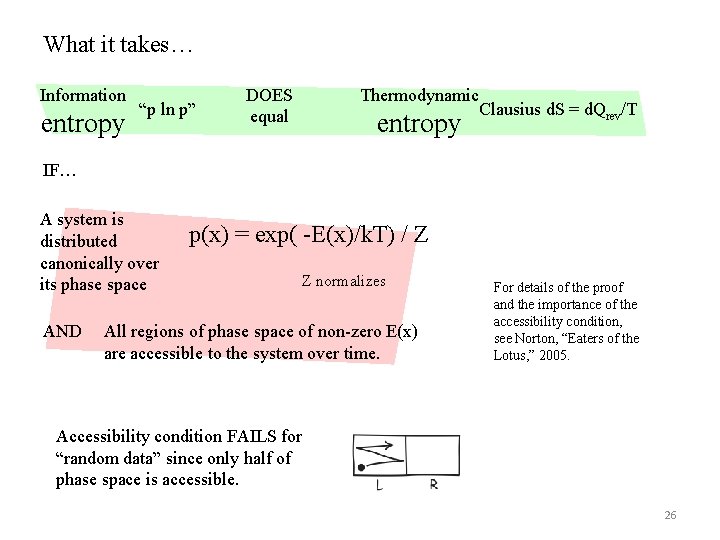
What it takes… Information entropy “p ln p” DOES equal Thermodynamic entropy Clausius d. S = d. Qrev/T IF… A system is distributed canonically over its phase space AND p(x) = exp( -E(x)/k. T) / Z Z normalizes All regions of phase space of non-zero E(x) are accessible to the system over time. For details of the proof and the importance of the accessibility condition, see Norton, “Eaters of the Lotus, ” 2005. Accessibility condition FAILS for “random data” since only half of phase space is accessible. 26

4. 27

Ladyman et al. , “The connection between logical and thermodynamic irreversibility, ” 2007. 4. An Indirect Proof One. Molecule gas insert partition or isothermal reversible expansion dissipationlessly Reduces entropy of heat bath by k ln 2. detect gas state One. Molecule memory Original proof given only in terms of quantities of heat passed among components. or shift cell to match perform any erasure Assume second law of thermodynamics holds on average. Erasure must create entropy k ln 2 on average. 28

4. An Indirect Proof Fails One. Molecule gas insert partition or dissipationlessly Reduces entropy of heat bath by k ln 2. detect gas state One. Molecule memory isothermal reversible expansion or shift cell to match Net effect is a reduction of entropy of heat bath. Second law violated even in statistical form. (Earman and Norton, 1999, “no-erasure” demon. ) Dissipationlessly detect memory state. If R, shift to L. Final step is a dissipationless erasure built out of processes routinely admitted in this literature. 29

The standard inventory of processes 30

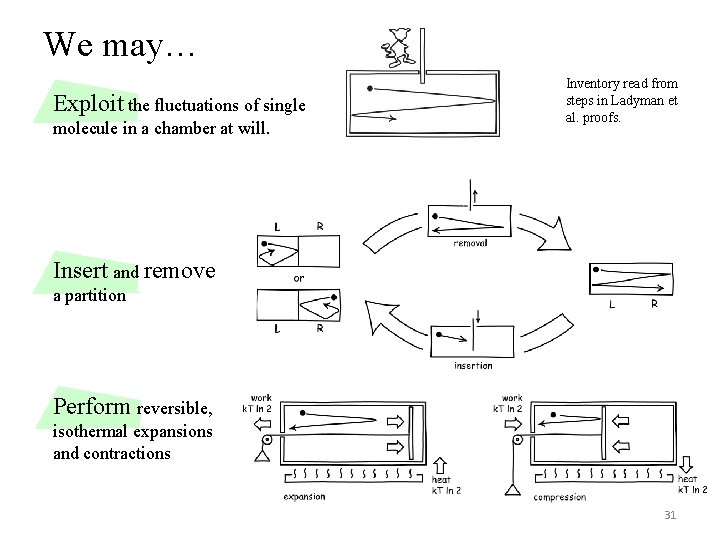
We may… Exploit the fluctuations of single molecule in a chamber at will. Inventory read from steps in Ladyman et al. proofs. Insert and remove a partition Perform reversible, isothermal expansions and contractions 31

We may… Detect the location of ? the molecule without dissipation. ? Shift between equal entropy states without dissipation. Memory Trigger new processes according to the location detected. R Gas ? L 32

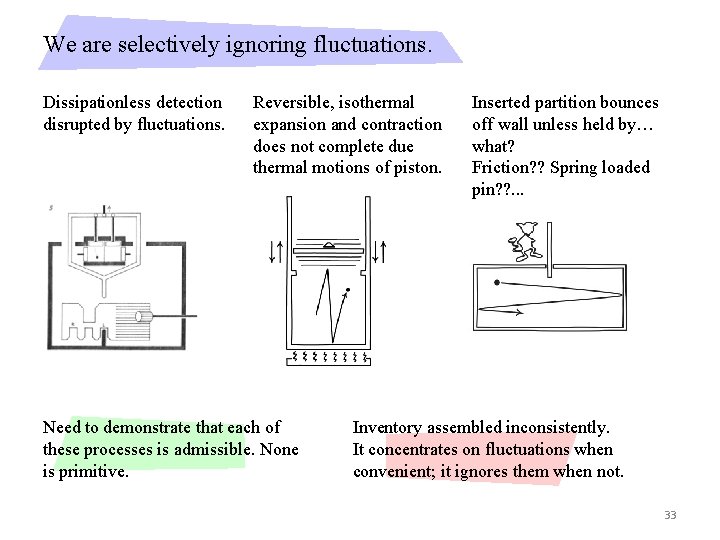
We are selectively ignoring fluctuations. Dissipationless detection disrupted by fluctuations. Reversible, isothermal expansion and contraction does not complete due thermal motions of piston. Need to demonstrate that each of these processes is admissible. None is primitive. Inserted partition bounces off wall unless held by… what? Friction? ? Spring loaded pin? ? . . . Inventory assembled inconsistently. It concentrates on fluctuations when convenient; it ignores them when not. 33

Conclusion 34

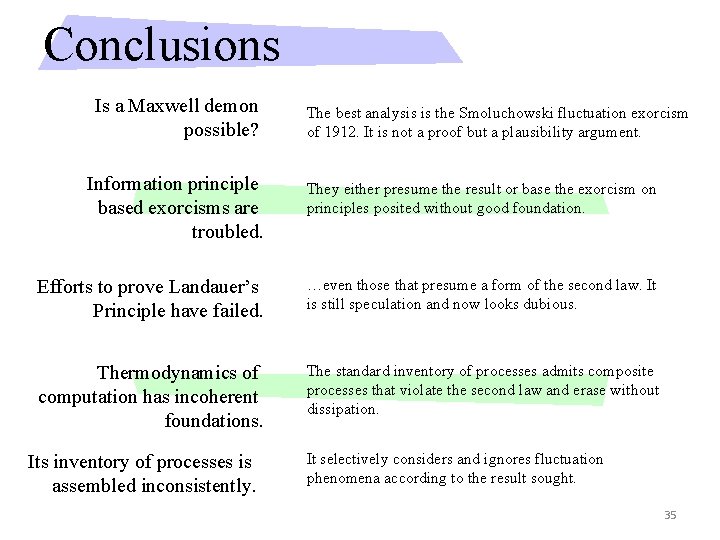
Conclusions Is a Maxwell demon possible? The best analysis is the Smoluchowski fluctuation exorcism of 1912. It is not a proof but a plausibility argument. Information principle based exorcisms are troubled. They either presume the result or base the exorcism on principles posited without good foundation. Efforts to prove Landauer’s Principle have failed. …even those that presume a form of the second law. It is still speculation and now looks dubious. Thermodynamics of computation has incoherent foundations. The standard inventory of processes admits composite processes that violate the second law and erase without dissipation. Its inventory of processes is assembled inconsistently. It selectively considers and ignores fluctuation phenomena according to the result sought. 35

Read all about it. 36

37

38

Commercials 39

40

Finis 41
 Chris passmore landauer
Chris passmore landauer What is information theory
What is information theory Intensive vs extensive properties
Intensive vs extensive properties Thermodynamic vs kinetic control
Thermodynamic vs kinetic control Mech
Mech Van't hoff equation
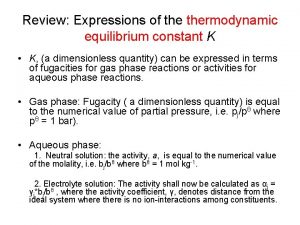
Van't hoff equation Thermodynamic equilibrium constant
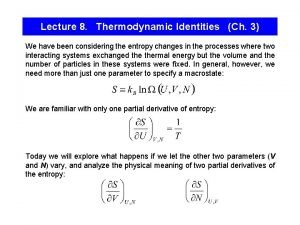
Thermodynamic equilibrium constant Thermodynamic identity
Thermodynamic identity Thermodynamic behaviour of ideal bose gas
Thermodynamic behaviour of ideal bose gas Cp-cv=r/m
Cp-cv=r/m Thermodynamic stability vs kinetic stability
Thermodynamic stability vs kinetic stability Thermodynamic property relations
Thermodynamic property relations Thermodynamics 1 formula sheet
Thermodynamics 1 formula sheet Thermodynamic
Thermodynamic Thermodynamic
Thermodynamic Volume expansivity and isothermal compressibility
Volume expansivity and isothermal compressibility Thermodynamic second law
Thermodynamic second law Thermodynamic behaviour of ideal bose gas
Thermodynamic behaviour of ideal bose gas Thermodynamic property relations
Thermodynamic property relations Etransl
Etransl Zeroth law example
Zeroth law example Second law of thermodynamic
Second law of thermodynamic Thermodynamic temperature
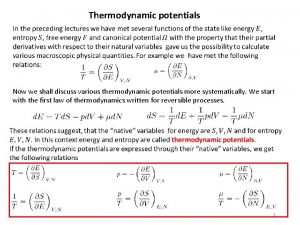
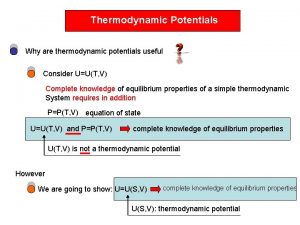
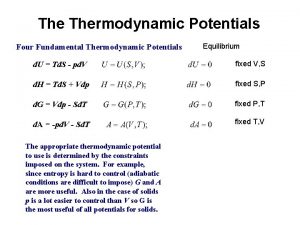
Thermodynamic temperature Thermodynamic potentials
Thermodynamic potentials Second law of thermodynamic
Second law of thermodynamic Entropy change formula
Entropy change formula Pericyclic
Pericyclic John wiley & sons, inc.
John wiley & sons, inc. Curtin-hammett principle
Curtin-hammett principle Thermodynamic square
Thermodynamic square Maxwell's thermodynamic relations
Maxwell's thermodynamic relations Gibbs free energy
Gibbs free energy Thermodynamic relations
Thermodynamic relations Thermodynamic
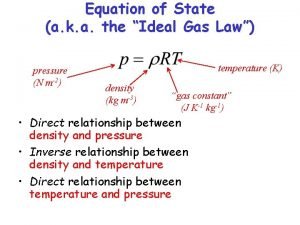
Thermodynamic Equation of state
Equation of state Enthalpy vs entropy
Enthalpy vs entropy Entropy and heat transfer
Entropy and heat transfer What is enthalpy and entropy
What is enthalpy and entropy Negative delta g
Negative delta g Enthalpy entropy free energy
Enthalpy entropy free energy Ap chemistry spontaneity entropy and free energy
Ap chemistry spontaneity entropy and free energy Minimum enthalpy and maximum entropy
Minimum enthalpy and maximum entropy