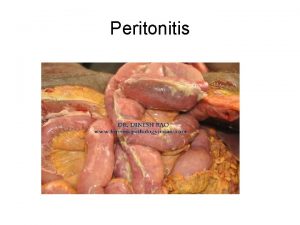
Infectious Complications of PD Peritonitis and Exit Site





- Slides: 36

Infectious Complications of PD: Peritonitis and Exit Site / Tunnel Infections Download Presentation at: www. pedpd. org Franz Schaefer Pediatric Nephrology Division Center for Pediatric and Adolescent Medicine University of Heidelberg, Germany

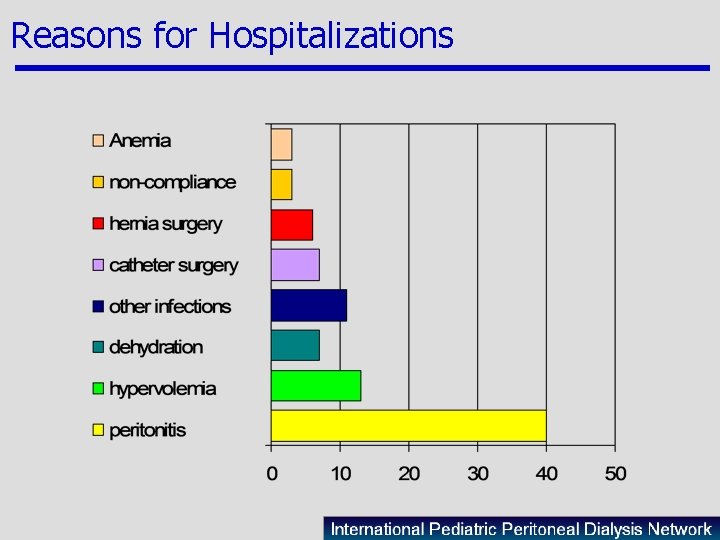
Reasons for Hospitalizations

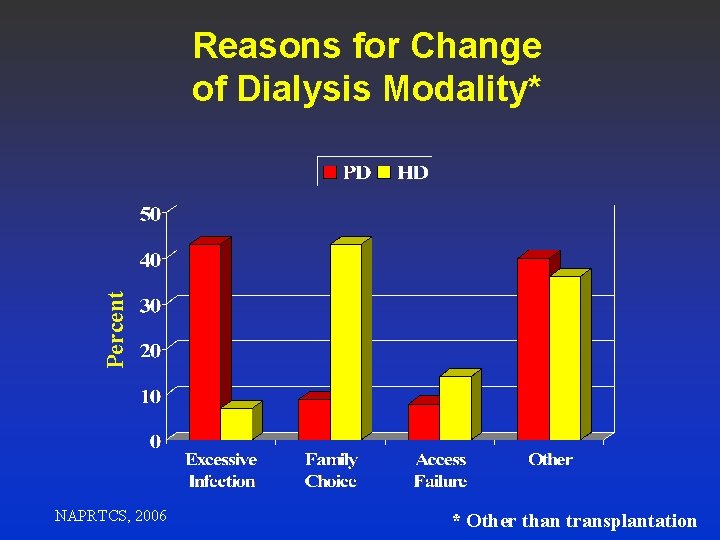
Percent Reasons for Change of Dialysis Modality* NAPRTCS, 2006 * Other than transplantation

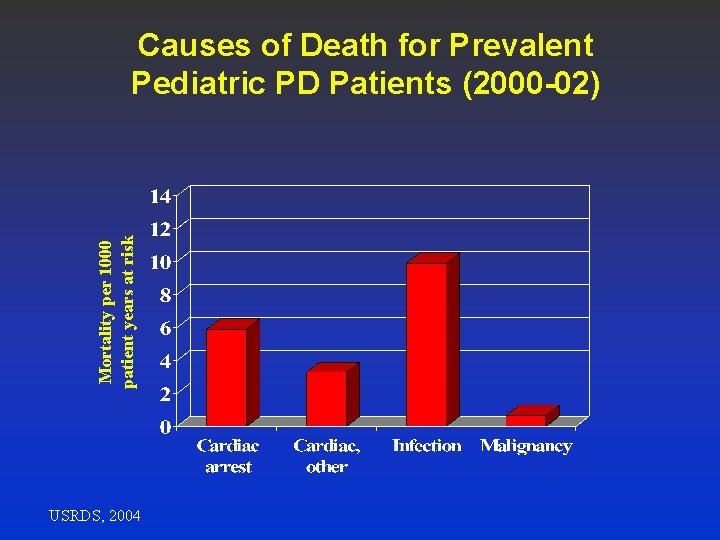
Mortality per 1000 patient years at risk Causes of Death for Prevalent Pediatric PD Patients (2000 -02) USRDS, 2004

www. peritonitis. org

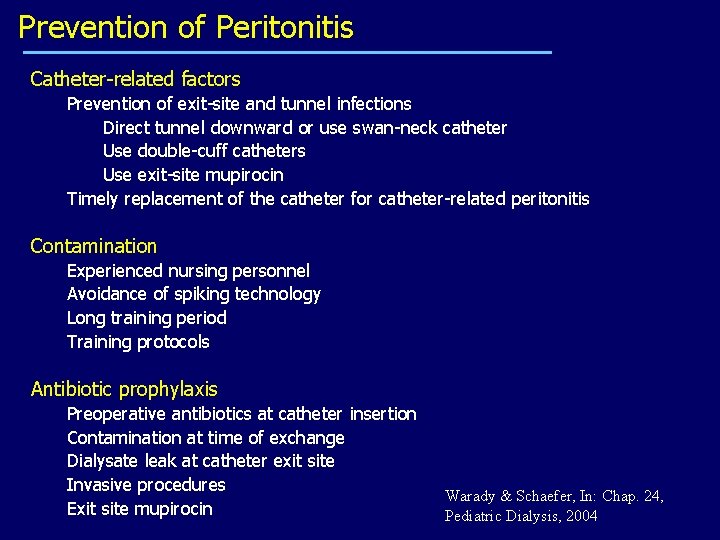
Prevention of Peritonitis Catheter-related factors Prevention of exit-site and tunnel infections Direct tunnel downward or use swan-neck catheter Use double-cuff catheters Use exit-site mupirocin Timely replacement of the catheter for catheter-related peritonitis Contamination Experienced nursing personnel Avoidance of spiking technology Long training period Training protocols Antibiotic prophylaxis Preoperative antibiotics at catheter insertion Contamination at time of exchange Dialysate leak at catheter exit site Invasive procedures Exit site mupirocin Warady & Schaefer, In: Chap. 24, Pediatric Dialysis, 2004

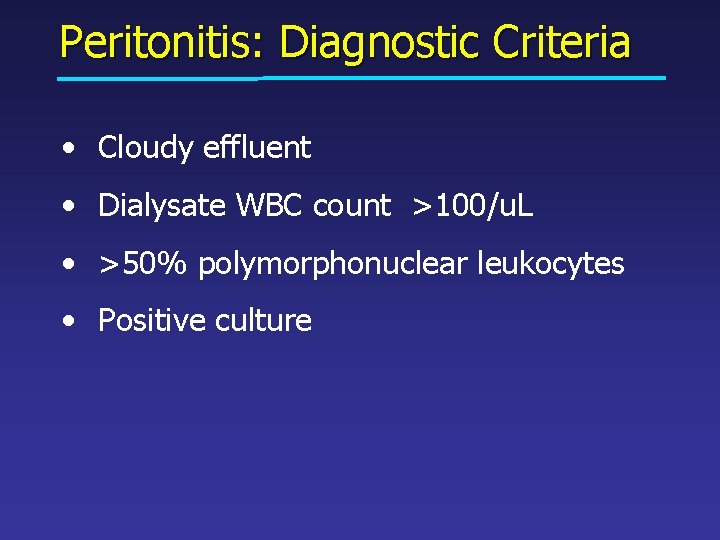
Peritonitis: Diagnostic Criteria • Cloudy effluent • Dialysate WBC count >100/u. L • >50% polymorphonuclear leukocytes • Positive culture

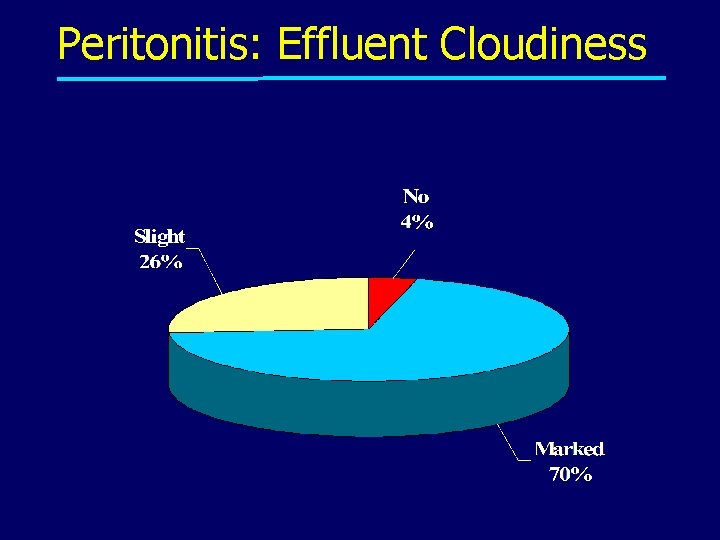
Peritonitis: Effluent Cloudiness


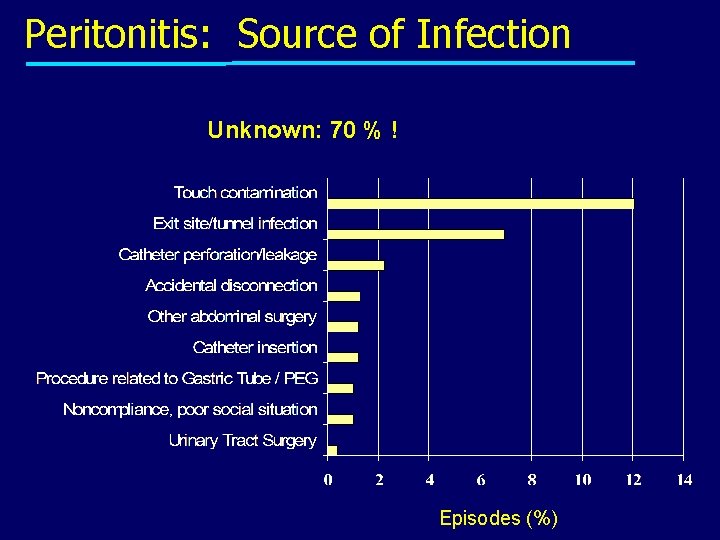
Peritonitis: Source of Infection Unknown: 70 % ! Episodes (%)

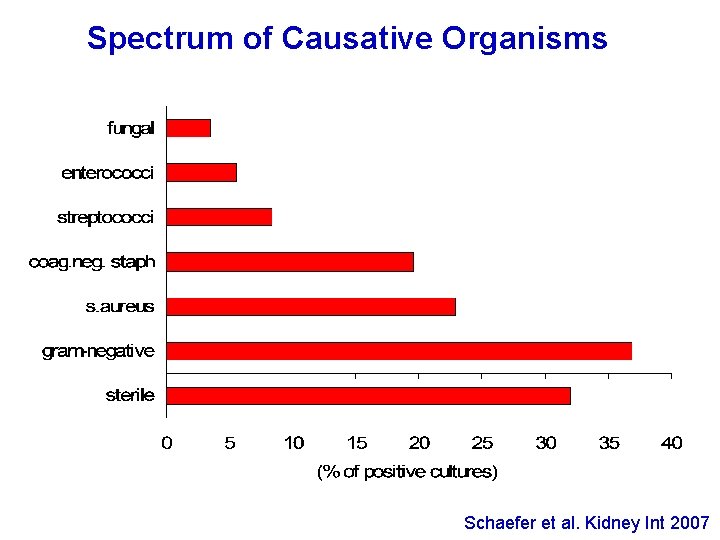
Spectrum of Causative Organisms Schaefer et al. Kidney Int 2007

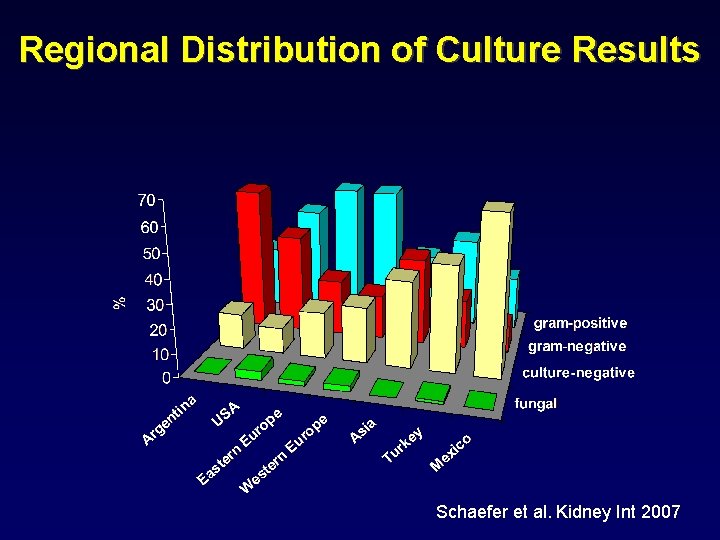
Regional Distribution of Culture Results Schaefer et al. Kidney Int 2007

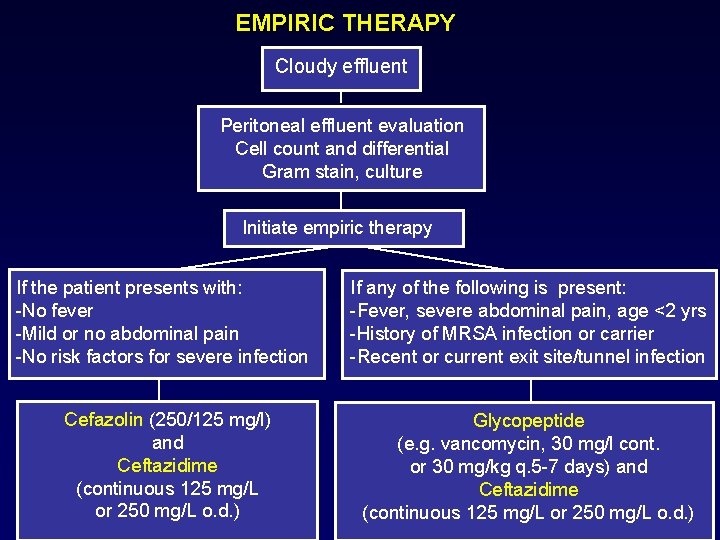
EMPIRIC THERAPY Cloudy effluent Peritoneal effluent evaluation Cell count and differential Gram stain, culture Initiate empiric therapy If the patient presents with: -No fever -Mild or no abdominal pain -No risk factors for severe infection If any of the following is present: -Fever, severe abdominal pain, age <2 yrs -History of MRSA infection or carrier -Recent or current exit site/tunnel infection Cefazolin (250/125 mg/l) and Ceftazidime (continuous 125 mg/L or 250 mg/L o. d. ) Glycopeptide (e. g. vancomycin, 30 mg/l cont. or 30 mg/kg q. 5 -7 days) and Ceftazidime (continuous 125 mg/L or 250 mg/L o. d. )

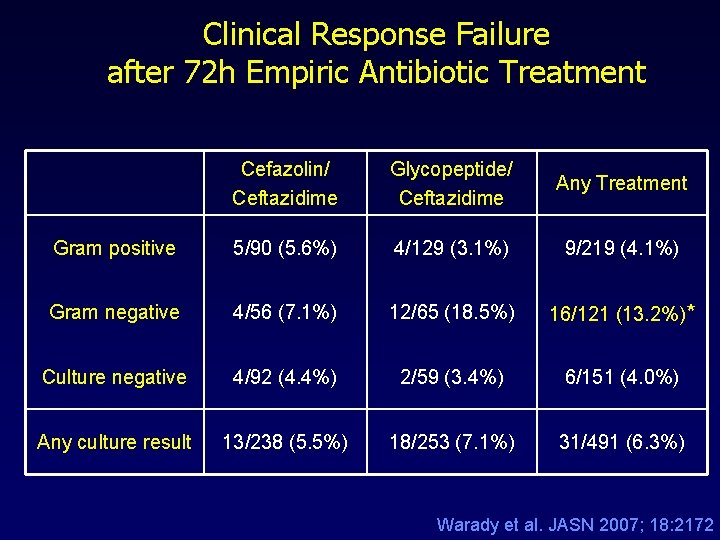
Clinical Response Failure after 72 h Empiric Antibiotic Treatment Cefazolin/ Ceftazidime Glycopeptide/ Ceftazidime Any Treatment Gram positive 5/90 (5. 6%) 4/129 (3. 1%) 9/219 (4. 1%) Gram negative 4/56 (7. 1%) 12/65 (18. 5%) 16/121 (13. 2%)* Culture negative 4/92 (4. 4%) 2/59 (3. 4%) 6/151 (4. 0%) Any culture result 13/238 (5. 5%) 18/253 (7. 1%) 31/491 (6. 3%) Warady et al. JASN 2007; 18: 2172

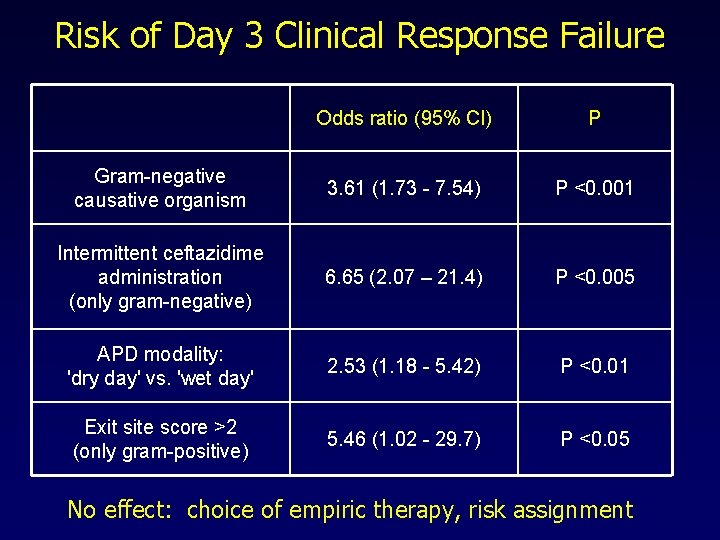
Risk of Day 3 Clinical Response Failure Odds ratio (95% Cl) P Gram-negative causative organism 3. 61 (1. 73 - 7. 54) P <0. 001 Intermittent ceftazidime administration (only gram-negative) 6. 65 (2. 07 – 21. 4) P <0. 005 APD modality: 'dry day' vs. 'wet day' 2. 53 (1. 18 - 5. 42) P <0. 01 Exit site score >2 (only gram-positive) 5. 46 (1. 02 - 29. 7) P <0. 05 No effect: choice of empiric therapy, risk assignment

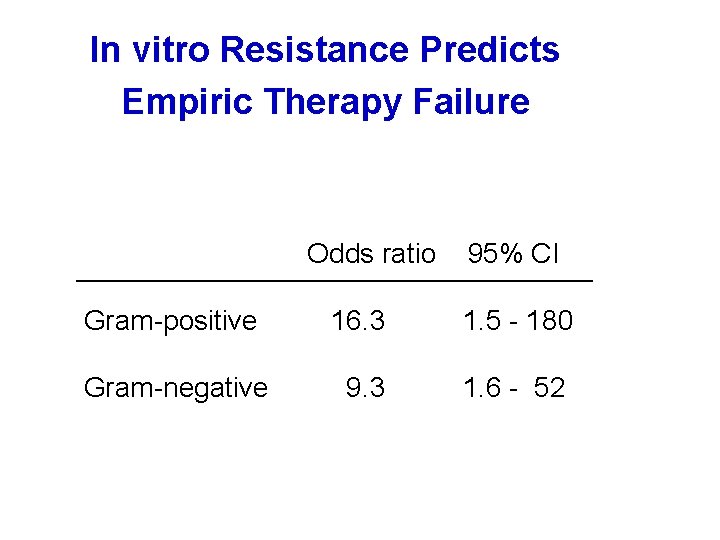
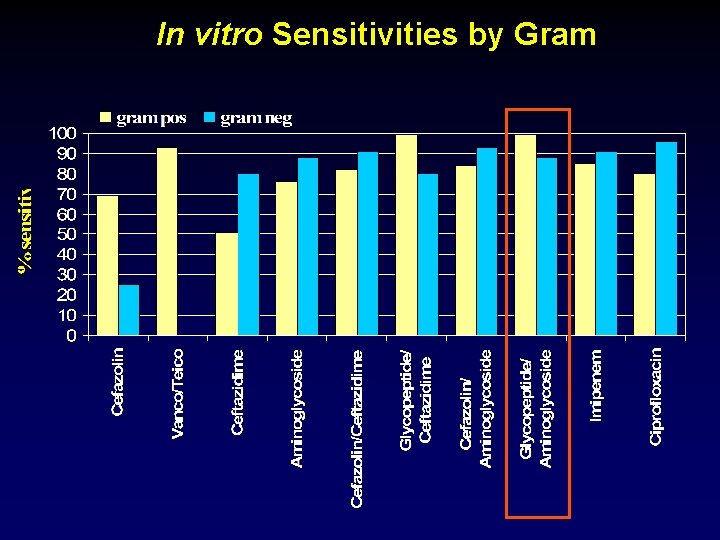
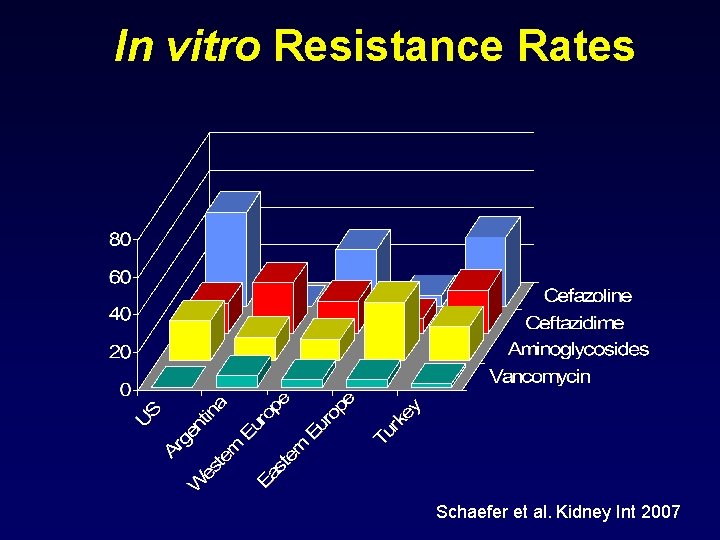
In vitro Resistance Predicts Empiric Therapy Failure Odds ratio 95% CI Gram-positive 16. 3 1. 5 - 180 Gram-negative 9. 3 1. 6 - 52

In vitro Sensitivities by Gram

In vitro Resistance Rates Schaefer et al. Kidney Int 2007

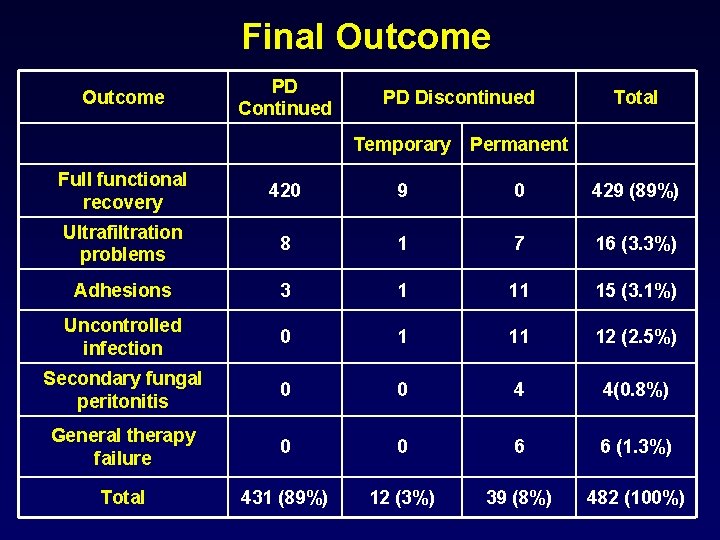
Final Outcome PD Continued PD Discontinued Total Temporary Permanent Full functional recovery 420 9 0 429 (89%) Ultrafiltration problems 8 1 7 16 (3. 3%) Adhesions 3 1 11 15 (3. 1%) Uncontrolled infection 0 1 11 12 (2. 5%) Secondary fungal peritonitis 0 0 4 4(0. 8%) General therapy failure 0 0 6 6 (1. 3%) Total 431 (89%) 12 (3%) 39 (8%) 482 (100%)

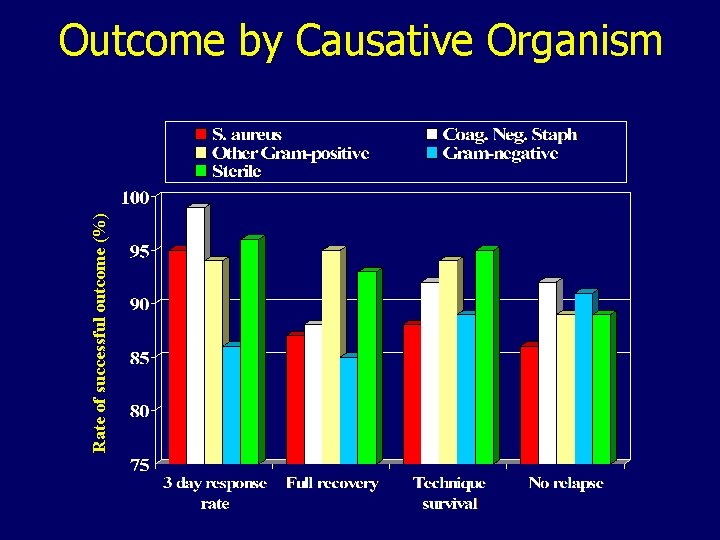
Rate of successful outcome (%) Outcome by Causative Organism

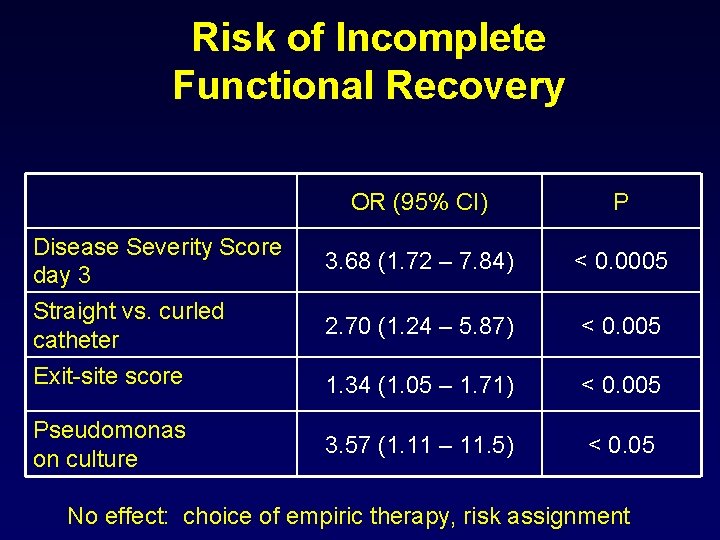
Risk of Incomplete Functional Recovery OR (95% CI) P 3. 68 (1. 72 – 7. 84) < 0. 0005 2. 70 (1. 24 – 5. 87) < 0. 005 Exit-site score 1. 34 (1. 05 – 1. 71) < 0. 005 Pseudomonas on culture 3. 57 (1. 11 – 11. 5) < 0. 05 Disease Severity Score day 3 Straight vs. curled catheter No effect: choice of empiric therapy, risk assignment

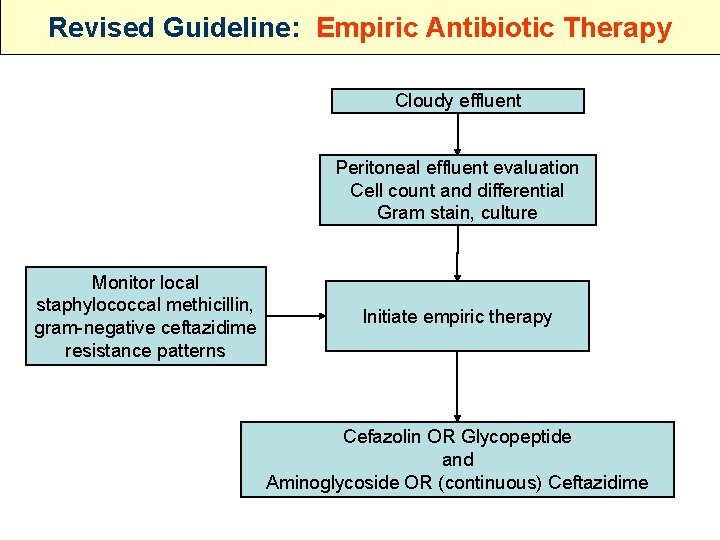
Revised Guideline: Empiric Antibiotic Therapy Cloudy effluent Peritoneal effluent evaluation Cell count and differential Gram stain, culture Monitor local staphylococcal methicillin, gram-negative ceftazidime resistance patterns Initiate empiric therapy Cefazolin OR Glycopeptide and Aminoglycoside OR (continuous) Ceftazidime

Revised Guideline: Modification for Culture Negative Episodes If improved clinically: Continue 1 st generation cephalosporin or glycopeptide for 14 days Discontinue aminoglycoside after 3 days Add/continue ceftazidime after 3 days If not improved clinically: Remove catheter

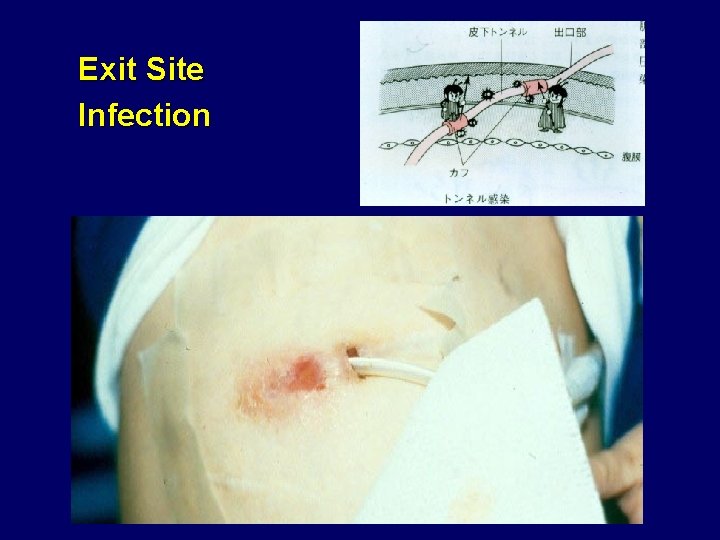
Exit Site Infection

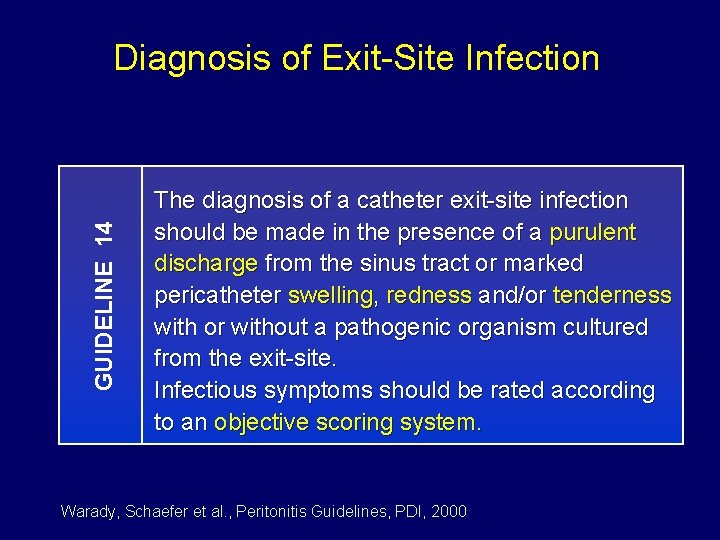
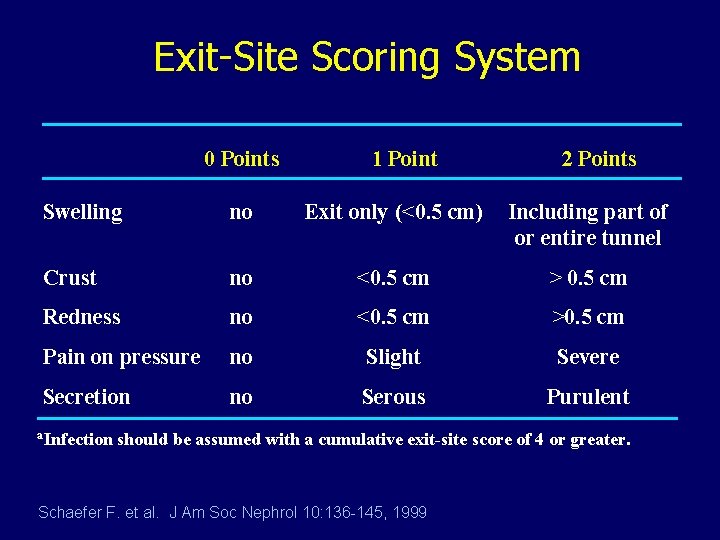
GUIDELINE 14 Diagnosis of Exit-Site Infection The diagnosis of a catheter exit-site infection should be made in the presence of a purulent discharge from the sinus tract or marked pericatheter swelling, redness and/or tenderness with or without a pathogenic organism cultured from the exit-site. Infectious symptoms should be rated according to an objective scoring system. Warady, Schaefer et al. , Peritonitis Guidelines, PDI, 2000

Exit-Site Scoring System 0 Points 1 Point 2 Points Swelling no Exit only (<0. 5 cm) Including part of or entire tunnel Crust no <0. 5 cm > 0. 5 cm Redness no <0. 5 cm >0. 5 cm Pain on pressure no Slight Severe Secretion no Serous Purulent a. Infection should be assumed with a cumulative exit-site score of 4 or greater. Schaefer F. et al. J Am Soc Nephrol 10: 136 -145, 1999

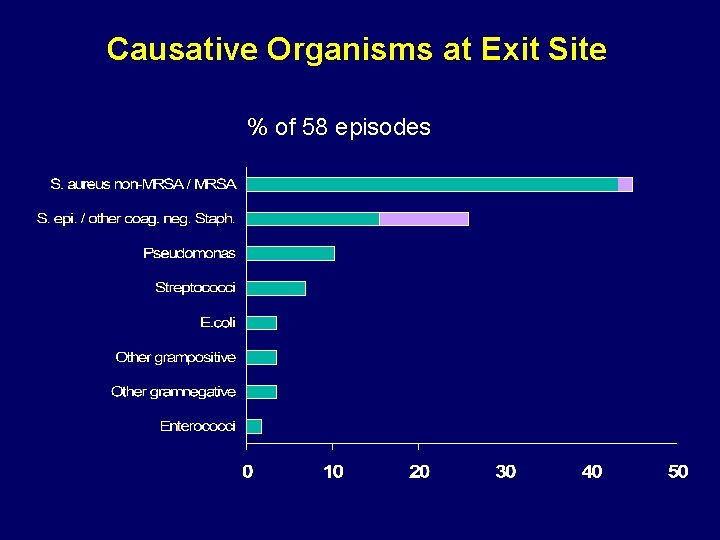
Causative Organisms at Exit Site % of 58 episodes

Therapy of Exit Site Infection • Usually oral • Usually upon culture results • Grampositive usually penicillinaseresistant penicillin or cefalexin • Length of therapy at least two weeks • One-stage catheter replacement for refractory ESI

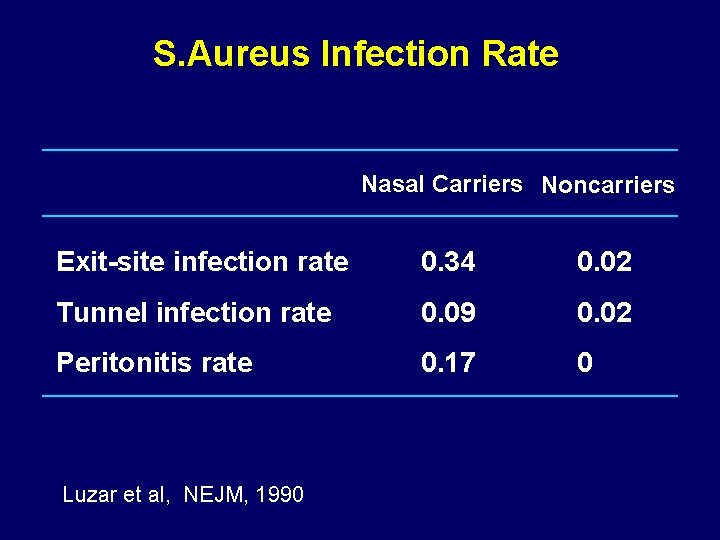
S. Aureus Infection Rate Nasal Carriers Noncarriers Exit-site infection rate 0. 34 0. 02 Tunnel infection rate 0. 09 0. 02 Peritonitis rate 0. 17 0 Luzar et al, NEJM, 1990

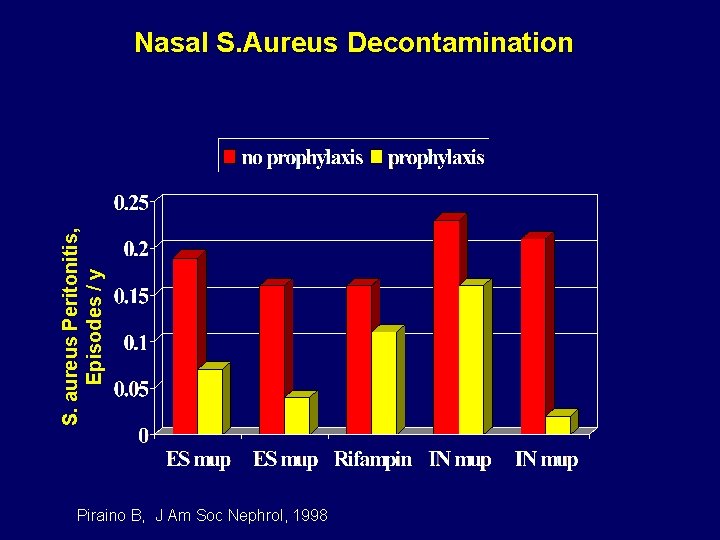
S. aureus Peritonitis, Episodes / y Nasal S. Aureus Decontamination Piraino B, J Am Soc Nephrol, 1998

Options for Prevention of Exit-Site Infections

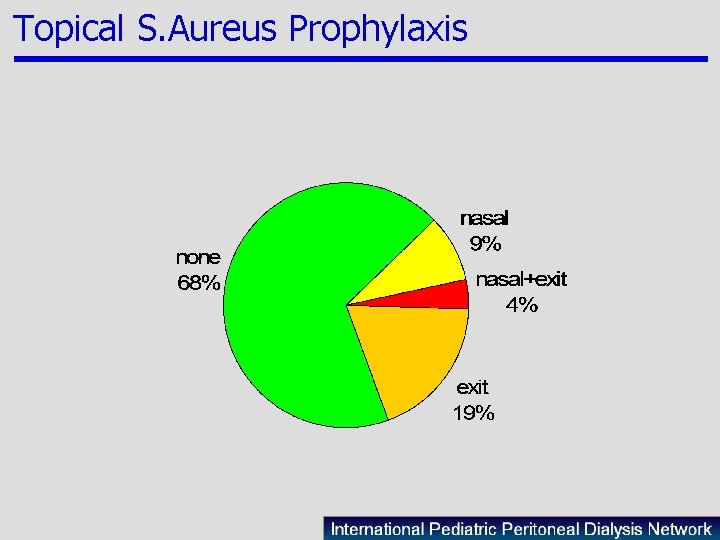
Topical S. Aureus Prophylaxis

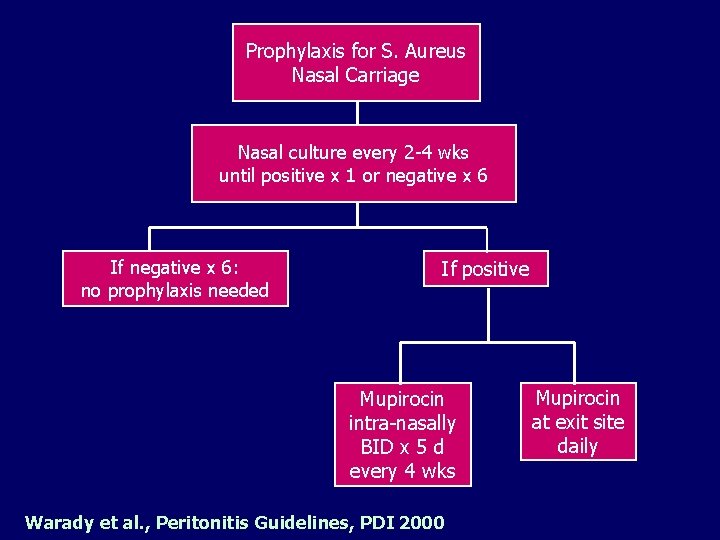
Prophylaxis for S. Aureus Nasal Carriage Nasal culture every 2 -4 wks until positive x 1 or negative x 6 If negative x 6: no prophylaxis needed If positive Mupirocin intra-nasally BID x 5 d every 4 wks Warady et al. , Peritonitis Guidelines, PDI 2000 Mupirocin at exit site daily

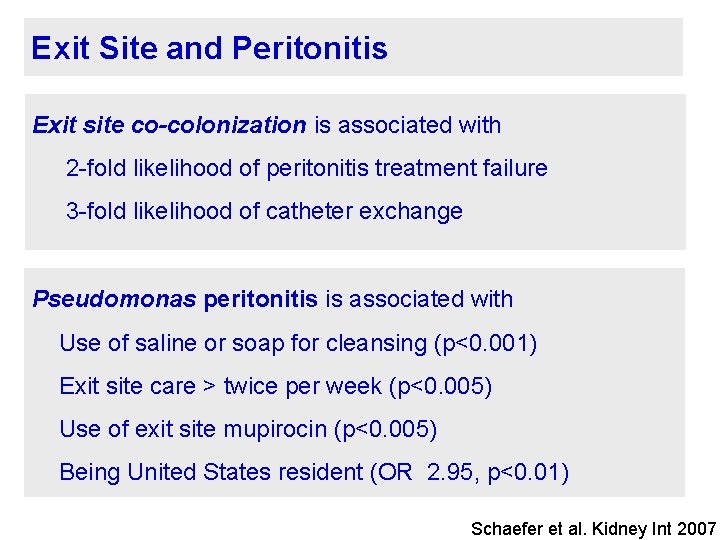
Exit Site and Peritonitis Exit site co-colonization is associated with 2 -fold likelihood of peritonitis treatment failure 3 -fold likelihood of catheter exchange Pseudomonas peritonitis is associated with Use of saline or soap for cleansing (p<0. 001) Exit site care > twice per week (p<0. 005) Use of exit site mupirocin (p<0. 005) Being United States resident (OR 2. 95, p<0. 01) Schaefer et al. Kidney Int 2007

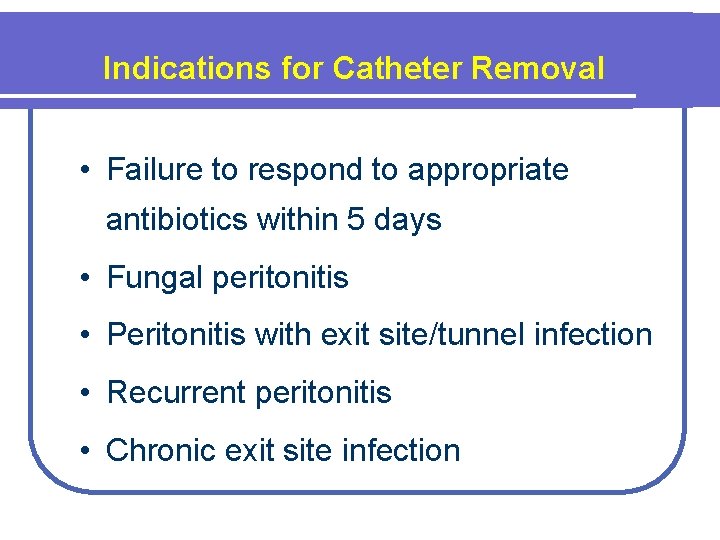
Indications for Catheter Removal • Failure to respond to appropriate antibiotics within 5 days • Fungal peritonitis • Peritonitis with exit site/tunnel infection • Recurrent peritonitis • Chronic exit site infection

International Pediatric PD Network www. pedpd. org
 Egress diagonal rule
Egress diagonal rule Exit exit access and exit discharge
Exit exit access and exit discharge Complication of intravenous therapy
Complication of intravenous therapy Iv site complications
Iv site complications Exit site scoring system
Exit site scoring system Peritonitis symptoms
Peritonitis symptoms Peritonitis refractaria
Peritonitis refractaria Cefotaxima oral
Cefotaxima oral Peritonitis bacteriana
Peritonitis bacteriana Nivel hidroaereo
Nivel hidroaereo Peritonitis symptoms
Peritonitis symptoms Peritonitis symptoms
Peritonitis symptoms Small intestine gangrene
Small intestine gangrene Darren tonkin
Darren tonkin Syphilis transmission
Syphilis transmission Tuberculoma
Tuberculoma Peritonitis
Peritonitis Caverna tbc
Caverna tbc Serositis
Serositis Peritonitis
Peritonitis Peritonitis radiopedia
Peritonitis radiopedia Topgl
Topgl Hot site cold site warm site disaster recovery
Hot site cold site warm site disaster recovery Chapter 26 infectious disease prevention and control
Chapter 26 infectious disease prevention and control Icd 10 morbus hansen
Icd 10 morbus hansen Poisonous and infectious material symbol
Poisonous and infectious material symbol Definition of infectious waste
Definition of infectious waste Infectious disease quality controls
Infectious disease quality controls Types of infection
Types of infection Stages of infectious disease
Stages of infectious disease Infectious canine hepatitis in dogs
Infectious canine hepatitis in dogs Infectious disease board review
Infectious disease board review What is the smallest infectious disease agent
What is the smallest infectious disease agent Papillomitosis
Papillomitosis Infectious nucleic acid
Infectious nucleic acid Infectious mononucleosis
Infectious mononucleosis Infectious disease
Infectious disease