Infection Control Cleaning Procedures Module 1 Part A















- Slides: 19

Infection Control Cleaning Procedures Module 1 Part A

Cleaning • Cleaning is a process by which microorganisms and biohazardous materials are removed from the surface of an object.

Cleaning is important because: • The effectiveness of the sterilisation / disinfection process is increased when there are less microorganisms to kill • All surfaces become fully exposed • Deactivating substances are removed • The working life of the item is prolonged • The item is clean and acceptable for the sterilisation / disinfection process

Control of microbe growth • Preventing organisms from gaining access to susceptible site • Killing all organisms • Reducing the number of microbes to an acceptable level • By exposing them to conditions that inhibit their growth

• Need enough time for all organisms to come into contact (recommended time) Rate of cell death differs • If there are larger numbers of microorganisms present the exposure time may have to be increased • Stage of growth – e. g. endospores (these are bacteria that are protected in a tough protective coat), cysts versus rapid growth • Chemical must be of prescribed strength (i. e. concentration)

Certain gram-positive bacilli are able to form endospores when environmental conditions are unfavourable. An endospore can survive conditions that destroy normal vegetative (growing) cells - such as boiling for up to five hours, freezing, desiccation (drying out), and exposure to chemicals and radiation Examples • Bacillus anthracis (anthrax) Endospore • Clostridium – • botulinum (botulism) • perfringens (food poisoning, gas gangrene) • tetani (tetanus)

• Cleaning - Equipment that does not pose significant risk to the patient from contact: beds, sinks, eating utensils Requirements for sterilisation and disinfection of equipment • Disinfection - (after cleaning) Equipment that comes into contact with patients' skin but doesn't penetrate: X-ray machines, bedpans, thermometers • Sterilisation - All equipment that penetrates the skin or mucous membranes: • Surgical instruments, needles (syringes), IV and urinary catheters, endoscopes. • All invasive devices, implants. • All solutions that are administered parenterally • All objects that contact a break in the skin or mucosal surfaces: dressings, swabs. • Surgical gowns, drapes and gloves

• The process of eliminating all micro-organisms except bacterial spores. • Disinfection CANNOT take the place of sterilisation for instruments used for invasive procedures Disinfection • Usually kills vegetative bacteria, not endospores or viruses • Kills (bactericide) or inactivates (bacteriostatic) large numbers of microorganisms • Must be able to decrease the number to an acceptable level • Chemical disinfectants that are mild enough to be used on the skin are called antiseptics

• Heat 1000 C - boiling water (5 mins) used for instruments, food • Pasteurisation - heating to 630 C - <1000 (30 mins) used for food, milk products Methods of disinfection • Radiation - Short wave UV used for surfaces, air spaces • Filtration – used for air in safety cabinets, operating rooms, isolation units, burns wards, ICU • Chemicals • Disinfectants used for surfaces, equipment, blood spills • Antiseptics used for decontamination of skin, hand washing

Pasteurisation Regulations Australia • The regulated minimum heat process for the holding time and temperature combination of milk pasteurisation in Australia is 72°C for 15 seconds. The Food Standards Code also allows for heating using any other time and temperature combination or process which has equal or greater lethal effect on any pathogenic bacteria. https: //www. dairysafe. vic. gov. au/publications-media/technical-information-notes/product/228 -htstpasteurisation

Phenolic • First was carbolic (Lister) it has a strong odour and is corrosive • Disrupts the cell wall, denature protein, inactivate enzymes Disinfectants • Remains active in the presence of organic material (e. g. pus, faeces) • Very effective against gram positive staph and strep • o-cresol is found in domestic disinfectants • Hexachlorophene - soaps, lotions, surgical scrubs, cosmetics, hygiene products

A carbolic spray may be used in the room with advantage during infectious Illness.

• Halogens i. e. chlorine and iodine • Iodophor - combination of povidone and iodine • Released slowly • Effective against C. perfringens (info next slide) Disinfectants • Can cause hypersensitivity • Pseudomonas can live in solution • Chlorine (sodium hypochlorite) - bleach • Inactivated by organic material • Compound breaks down in sunlight • 10, 000 ppm (parts per million) i. e. 1% for spillages

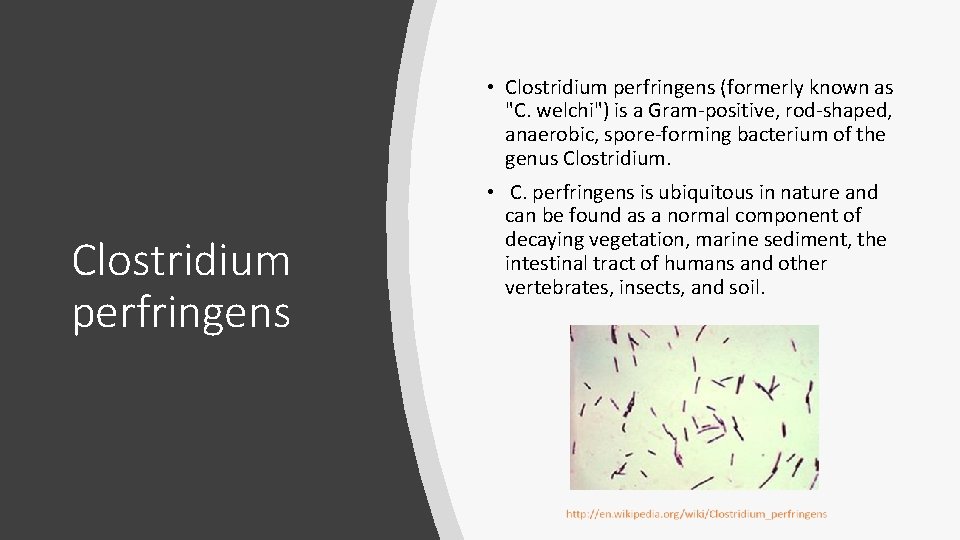
• Clostridium perfringens (formerly known as "C. welchi") is a Gram-positive, rod-shaped, anaerobic, spore-forming bacterium of the genus Clostridium perfringens • C. perfringens is ubiquitous in nature and can be found as a normal component of decaying vegetation, marine sediment, the intestinal tract of humans and other vertebrates, insects, and soil.

• Chlorhexidine in combination with soaps and detergents • As alcohol wipes/skin lotions • Doesn't affect mycobacterium or endospores • Pseudomonas can live in aqueous solutions Disinfectants • Low toxicity

• Alcohol - ethyl, isopropyl - most effective when mixed with water (the water helps it to penetrate the cell wall) • Effective against many microbes • Often used in combination with other antiseptics Disinfectants • Presence of organic material affects effectiveness • They denature proteins, dissolve lipids in cell walls Isopropyl Alcohol Cleaning Pads - Pk. 10. . . jaycar. com. au

• Quaternary ammonia compounds • Benzyl alkonium chloride- preservative in eye drops • Cetylpyridinium chloride - oral preparations such as antibacterial lozenges and mouthwashes Disinfectants • Effective against most bacteria, also fungi, some protozoa and enveloped viruses • Affect cell wall

Next Sterilisation • To complete this Course please download the next follow on lesson on Sterilisation Module 1 Part B

The End