Industrial Inorganic Chemistry and Laboratory JungWon Kim Department

Industrial Inorganic Chemistry and Laboratory Jung-Won Kim Department of Chemical Engineering Kangwon National University J

Contents Chap. 2 Crystal Structure 2. 1 Introduction for Crystal Structure 2. 2 Na. Cl Type Crystal Structure 2. 3 Perovskite Type Crystal Structure 2. 4 Spinel Type Crystal Structure 2. 5 Fluorite Type Crystal Structure 2. 6 Rutile Type Crystal Structure 2. 7 Layer Structure
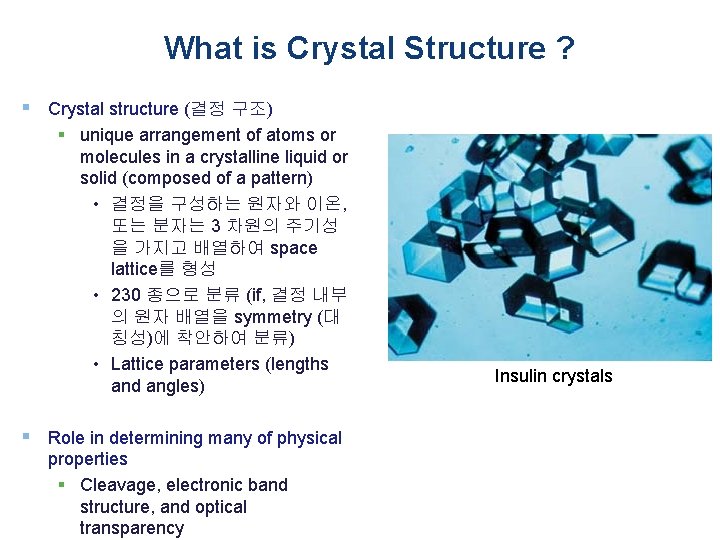
What is Crystal Structure ? § Crystal structure (결정 구조) § unique arrangement of atoms or molecules in a crystalline liquid or solid (composed of a pattern) • 결정을 구성하는 원자와 이온, 또는 분자는 3 차원의 주기성 을 가지고 배열하여 space lattice를 형성 • 230 종으로 분류 (if, 결정 내부 의 원자 배열을 symmetry (대 칭성)에 착안하여 분류) • Lattice parameters (lengths and angles) § Role in determining many of physical properties § Cleavage, electronic band structure, and optical transparency Insulin crystals
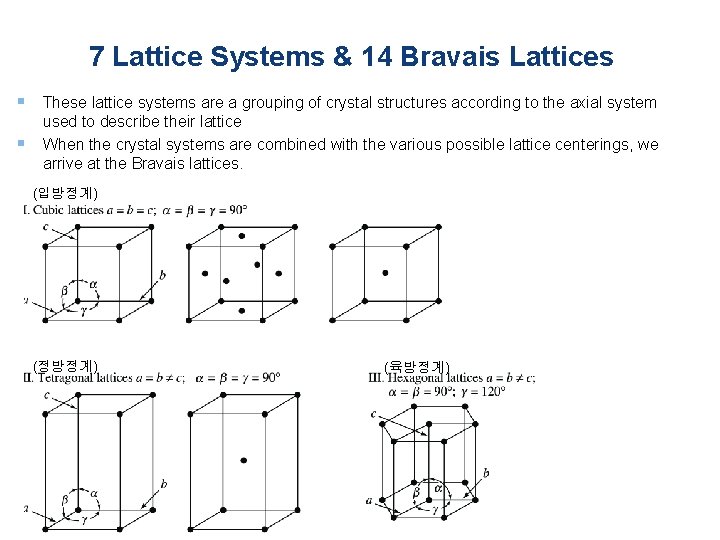
7 Lattice Systems & 14 Bravais Lattices § These lattice systems are a grouping of crystal structures according to the axial system § used to describe their lattice When the crystal systems are combined with the various possible lattice centerings, we arrive at the Bravais lattices. (입방정계) (정방정계) (육방정계)
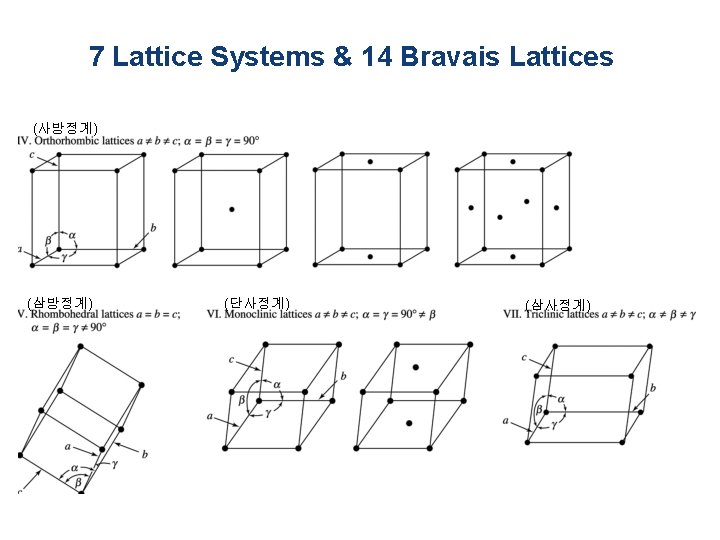
7 Lattice Systems & 14 Bravais Lattices (사방정계) (삼방정계) (단사정계) (삼사정계)

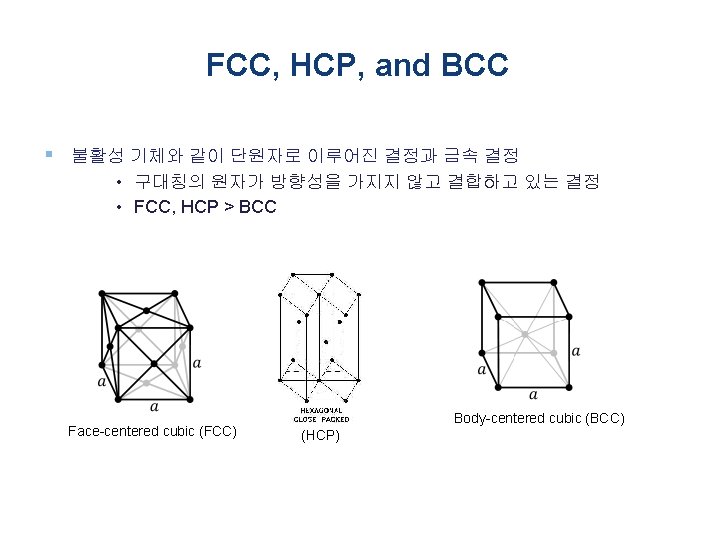
7 Lattice Systems & 14 Bravais Lattices (입방정계) Simple cubic (SC) Face-centered cubic (FCC) Body-centered cubic (BCC)

7 Lattice Systems & 14 Bravais Lattices (육방정계) In a hexagonal close-packed (HCP) arrangement of atoms, the unit cell consists of three layers of atoms. The top and bottom layers contain six atoms at the corners of a hexagon and one atom at the center of each hexagon. The middle layer contains three atoms nestled between the atoms of the top and bottom layers, hence, the name close-packed. (단사정계, Monoclinic lattices) Simple cubic (SC) Base-centered (HCP)

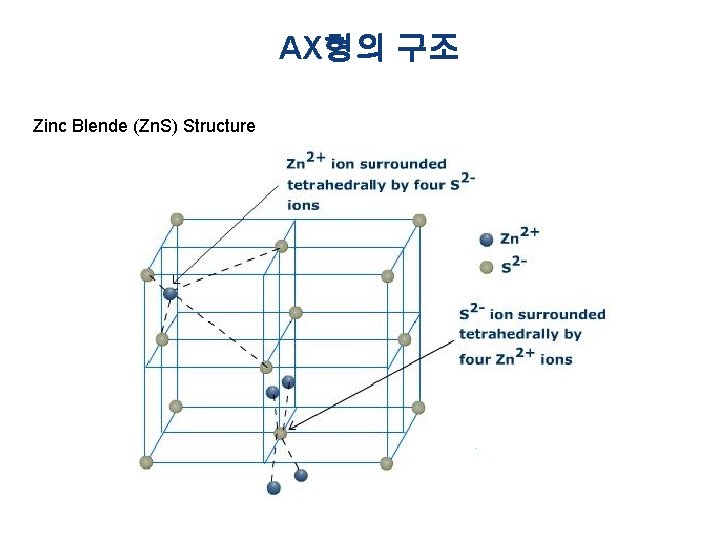
AX형의 구조 Zinc Blende (Zn. S) Structure

AX형의 구조 § Ag. I (Silver iodide) : The crystalline structure adopted by silver iodide changes with temperature. - Up to 420 K (147 °C), Ag. I exists in the β-phase, which has a wurtzite structure - Above 420 K (147 °C), Ag. I undergoes a transition to the α-phase, which has a body-centered cubic structure and has the silver ions distributed randomly between 2 -, 3 -, and 4 -coordinate sites. - A metastable γ-phase also exists below 420 K, which has a zinc blende structure.
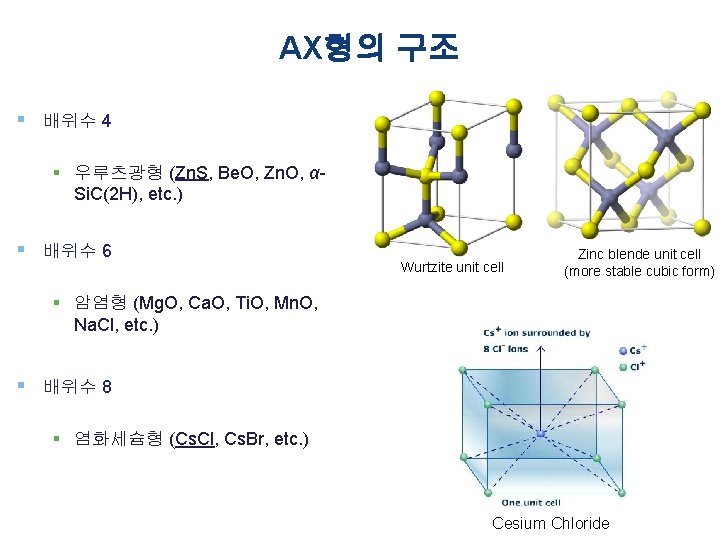
AX형의 구조 § 배위수 4 § 우루츠광형 (Zn. S, Be. O, Zn. O, αSi. C(2 H), etc. ) § 배위수 6 Wurtzite unit cell Zinc blende unit cell (more stable cubic form) § 암염형 (Mg. O, Ca. O, Ti. O, Mn. O, Na. Cl, etc. ) § 배위수 8 § 염화세슘형 (Cs. Cl, Cs. Br, etc. ) Cesium Chloride
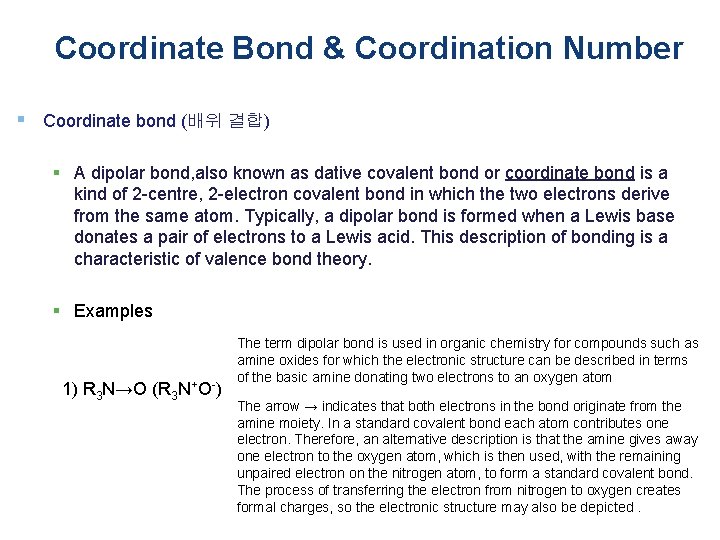
Coordinate Bond & Coordination Number § Coordinate bond (배위 결합) § A dipolar bond, also known as dative covalent bond or coordinate bond is a kind of 2 -centre, 2 -electron covalent bond in which the two electrons derive from the same atom. Typically, a dipolar bond is formed when a Lewis base donates a pair of electrons to a Lewis acid. This description of bonding is a characteristic of valence bond theory. § Examples 1) R 3 N→O (R 3 N+O-) The term dipolar bond is used in organic chemistry for compounds such as amine oxides for which the electronic structure can be described in terms of the basic amine donating two electrons to an oxygen atom The arrow → indicates that both electrons in the bond originate from the amine moiety. In a standard covalent bond each atom contributes one electron. Therefore, an alternative description is that the amine gives away one electron to the oxygen atom, which is then used, with the remaining unpaired electron on the nitrogen atom, to form a standard covalent bond. The process of transferring the electron from nitrogen to oxygen creates formal charges, so the electronic structure may also be depicted.
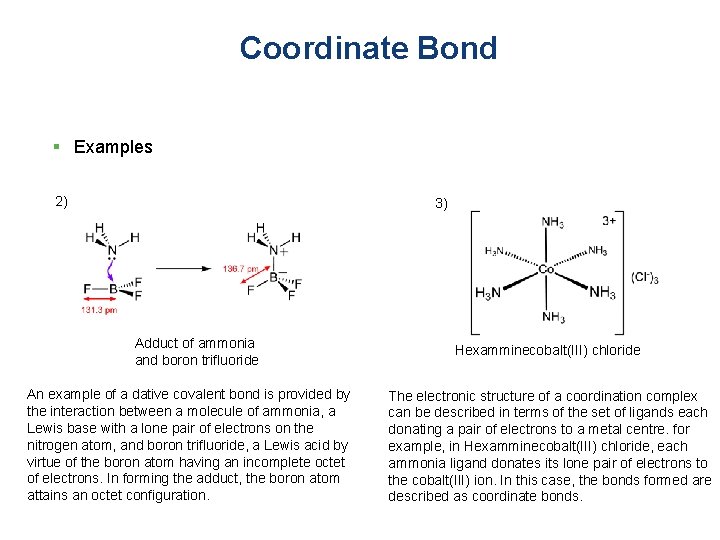
Coordinate Bond § Examples 2) 3) Adduct of ammonia and boron trifluoride An example of a dative covalent bond is provided by the interaction between a molecule of ammonia, a Lewis base with a lone pair of electrons on the nitrogen atom, and boron trifluoride, a Lewis acid by virtue of the boron atom having an incomplete octet of electrons. In forming the adduct, the boron atom attains an octet configuration. Hexamminecobalt(III) chloride The electronic structure of a coordination complex can be described in terms of the set of ligands each donating a pair of electrons to a metal centre. for example, in Hexamminecobalt(III) chloride, each ammonia ligand donates its lone pair of electrons to the cobalt(III) ion. In this case, the bonds formed are described as coordinate bonds.

Coordination Number § Coordination # (배위수) § In chemistry and crystallography, the coordination number of a central atom in a molecule or crystal is the number of its nearest neighbours. This number is determined somewhat differently for molecules and for crystals. § In chemistry the emphasis is on bonding structure in molecules or ions and the coordination number of an atom is determined by simply counting the other atoms to which it is bonded (by either single or multiple bonds). For example, [Cr(NH 3)6]Cl 3 has Cr 3+ as its central cation, which has a coordination number of 6.
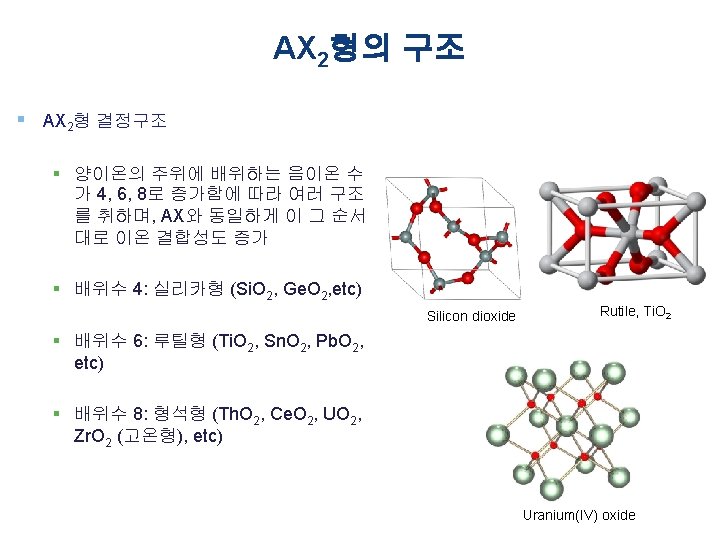
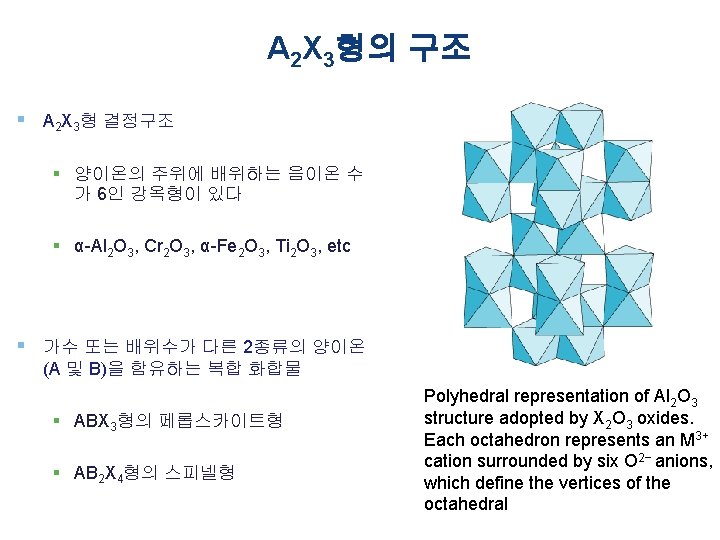
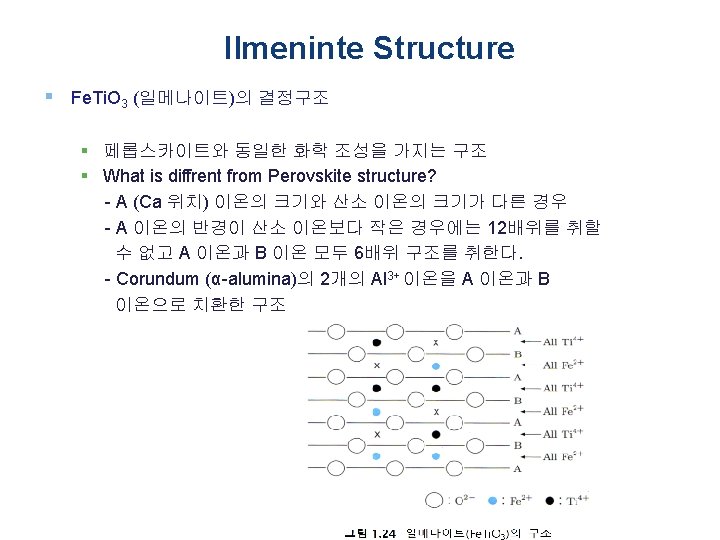
A 2 X 3형의 구조 § A 2 X 3형 결정구조 § 양이온의 주위에 배위하는 음이온 수 가 6인 강옥형이 있다 § α-Al 2 O 3, Cr 2 O 3, α-Fe 2 O 3, Ti 2 O 3, etc § 가수 또는 배위수가 다른 2종류의 양이온 (A 및 B)을 함유하는 복합 화합물 § ABX 3형의 페롭스카이트형 § AB 2 X 4형의 스피넬형 Polyhedral representation of Al 2 O 3 structure adopted by X 2 O 3 oxides. Each octahedron represents an M 3+ cation surrounded by six O 2− anions, which define the vertices of the octahedral

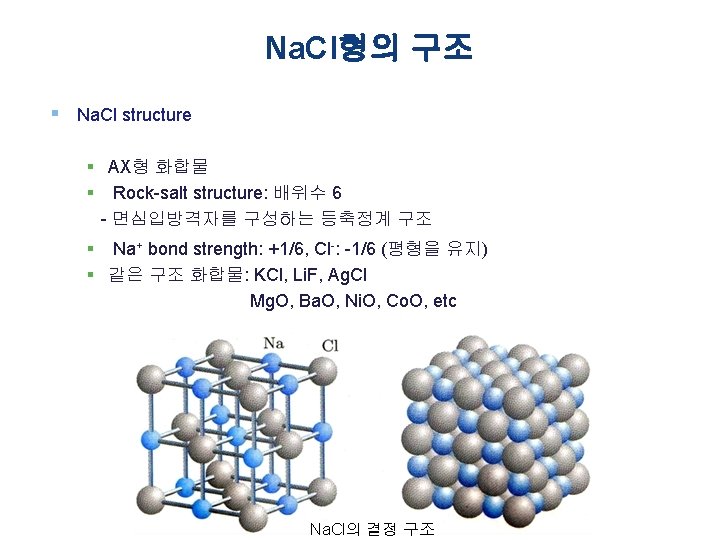
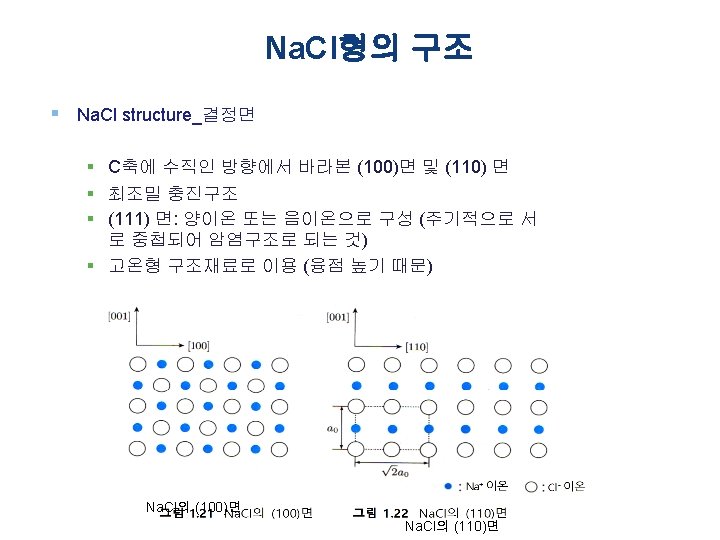
Chap. 2 2. 2 Na. Cl Type Crystal Structure

Chap. 2 2. 3 Perovskite Type Crystal Structure

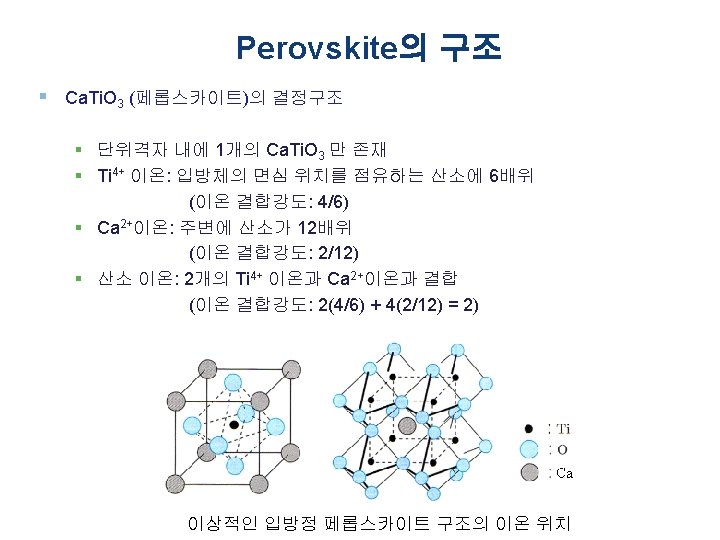
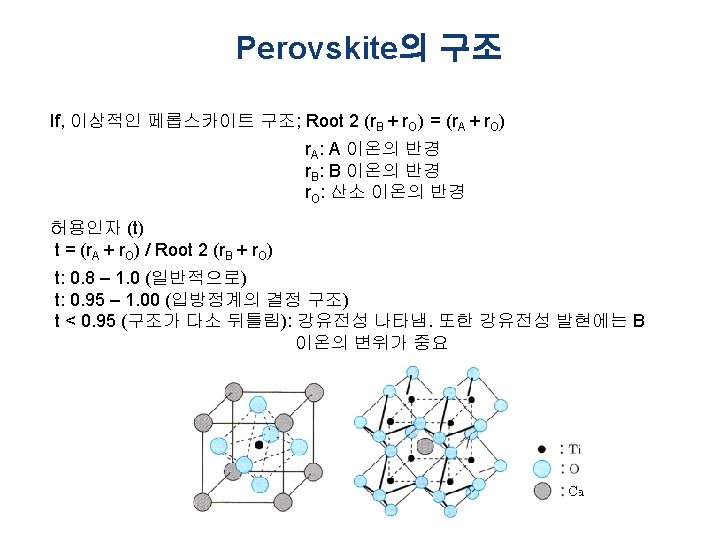
Perovskite의 구조 § Ca. Ti. O 3 (페롭스카이트)의 결정구조 § Perovskite (Pv) is a calcium titanium oxide mineral species composed of calcium titanate, with the chemical formula Ca. Ti. O 3 (부도체, 반도체, 도체의 성질 은 물론 초전도 현상까지 보이는 특별한 구조의 금속산 화물) § The mineral was discovered in the Ural mountains of Russia by Gustav Rose in 1839 and is named after Russian mineralogist L. A. Perovski (1792– 1856) § ABO 3 형: Ba. Ti. O 3 & Pb(Ti, Zr)O 3 (전자부품용 재료) § 다양한 물리성질을 갖고 있어 차세대 DRAM, 차세대 비 휘발성 메모리재료, 연료전지 전극재료 등 중요한 분야 에 응용

Chap. 2 2. 4 Spinel Type Crystal Structure
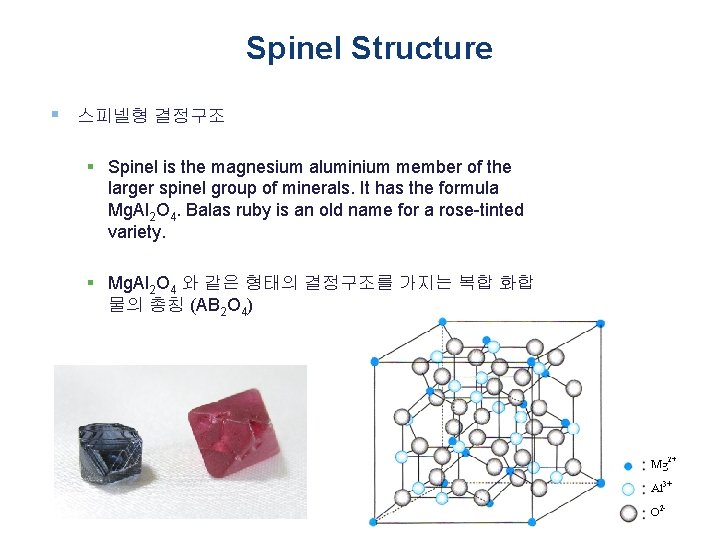
Spinel Structure § 스피넬형 결정구조 § Spinel is the magnesium aluminium member of the larger spinel group of minerals. It has the formula Mg. Al 2 O 4. Balas ruby is an old name for a rose-tinted variety. § Mg. Al 2 O 4 와 같은 형태의 결정구조를 가지는 복합 화합 물의 총칭 (AB 2 O 4)
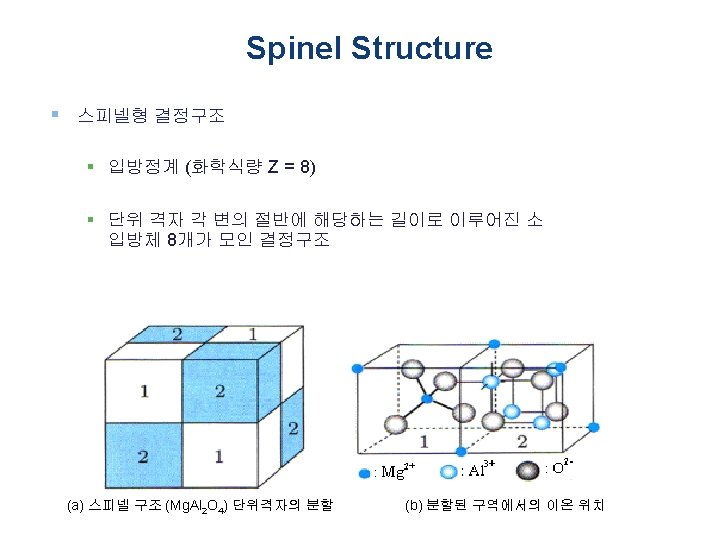
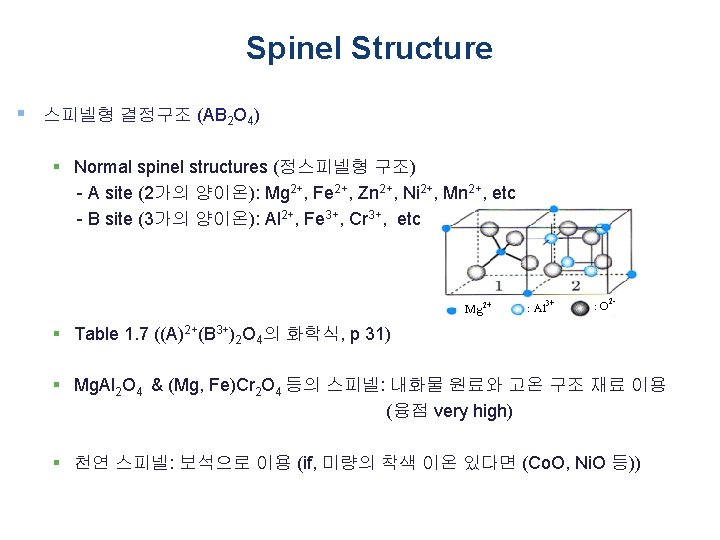

Spinel Structure § 스피넬형 결정구조 (AB 2 O 4) § Inverse spinel structures (역스피넬형 구조) - B site에 들어가야 되는 양이온의 절반이 A site에 들어가고, B site에는 나머지 B site 에 들어가야 하는 양이온과 A site에 들어가야 하는 양이온이 혼재하여 조성식이 B(AB)O 4로 되는 화합물 § Table 1. 7 ((A)4+(B 2+)2 O 4의 화학식, p 31) § Ferrite (자성체) - Fe를 함유하는 스피넬 - 강한 자성(페리 자성)을 나타내기 때문에 자속으로 이용 - Fe 2 O 3 or Fe. O·Fe 2 O 3 (마그네타이트): 4 배위의 위치에 들어가는 Fe 2+ 이온과 6 배위 위치에 들어가는 Fe 3+ 이온이 있다. 각각의 spin direction은 반대. (A common example of an inverse spinel is Fe 3 O 4, if the Fe 2+ (A 2+ ) ions are d 6 high-spin and the Fe 3+ (B 3+) ions are d 5 high-spin) - The magnetic material known as "Zn. Fe" has the formula Zn. Fe 2 O 4, with Fe 3+ occupying the octahedral sites and half of the tetrahedral sites. The remaining tetrahedral sites in this spinel are occupied by Zn 2+. (Zn. Fe 2 O 4는 Normal spinel structure)

Spinel Structure § 스피넬형 결정구조 (AB 2 O 4) § The spinels are any of a class of minerals of general formulation A 2+B 3+2 O 2 -4 which crystallise in the cubic (isometric) crystal system, with the oxide anions arranged in a cubic close-packed lattice and the cations A and B occupying some or all of the octahedral and tetrahedral sites in the lattice. § A and B can be divalent, trivalent, or quadrivalent cations, including magnesium, zinc, iron, manganese, aluminium, chromium, titanium, and silicon. § Although the anion is normally oxide, the analogous thiospinel structure includes the rest of the chalcogenides. § A and B can also be the same metal under different charges, such as the case in Fe 3 O 4 (as Fe 2+Fe 3+2 O 2 -4).
Spinel Structure § Members of the spinel group(AB 2 O 4) include § Aluminium spinels: - Spinel: Mg. Al 2 O 4, after which this class of minerals is named - Gahnite: Zn. Al 2 O 4 - Hercynite: Fe. Al 2 O 4 § Iron spinels: - Cuprospinel: Cu. Fe 2 O 4 - Franklinite: (Fe, Mn, Zn)(Fe, Mn) 2 O 4 - Jacobsite: Mn. Fe 2 O 4 - Magnetite: Fe 3 O 4 - Trevorite: Ni. Fe 2 O 4 - Ulvöspinel: Ti. Fe 2 O 4 - Zinc ferrite: (Zn, Fe) Fe 2 O 4 Gahnite, Zn. Al 2 O 4, is a rare mineral belonging to the spinel group Magnetite is a ferrimagnetic mineral with chemical formula Fe 3 O 4, one of several iron oxides and a member of the spinel group
Spinel Structure § Members of the spinel group(AB 2 O 4) include § Chromium spinels: - Chromite: Fe. Cr 2 O 4 - Magnesiochromite: Mg. Cr 2 O 4 Chromite is an iron chromium oxide: Fe. Cr 2 O 4. It is an oxide mineral belonging to the spinel group. § Others with the spinel structure: - Ringwoodite: (Mg, Fe) 2 Si. O 4, an abundant olivine polymorph within the Earth's mantle from about 520 to 660 km depth, and a rare mineral in meteorites § There are many more compounds with a spinel structure, e. g. the thiospinels and selenospinels, that can either be synthesized in the lab or in some cases occur as minerals.

Chap. 2 2. 5 Fluorite Type Crystal Structure

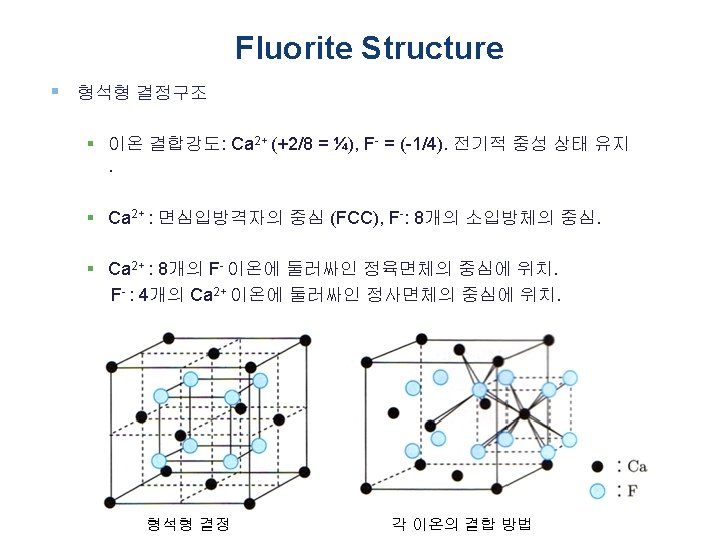
Fluorite Structure § 형석형 결정구조 § Fluorite is a halide mineral composed of calcium fluoride, Ca. F 2. § 이온 반경: Ca 2+ = 0. 112 nm, F- = 0. 131 nm (반경비: 0. 85, Ca 2+ 이 온 8배위로 안정) F Ca. F 2 형석형 결정 Ca


Chap. 2 2. 6 Rutile Type Crystal Structure
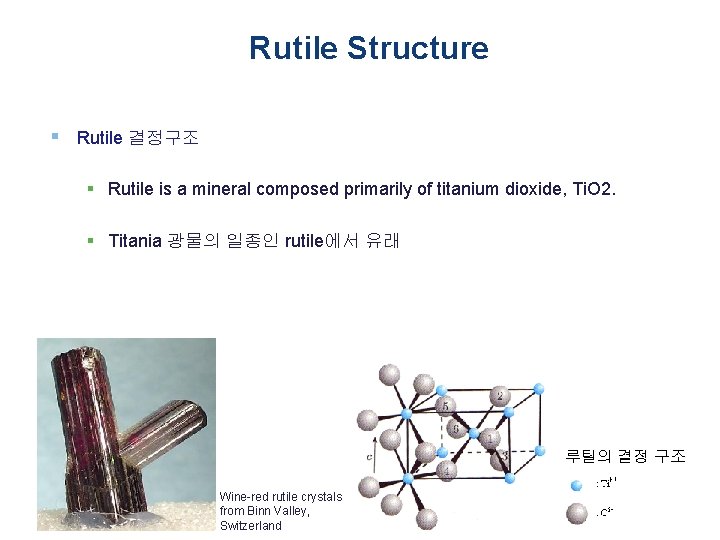
Rutile Structure § Rutile 결정구조 § Rutile is a mineral composed primarily of titanium dioxide, Ti. O 2. § Titania 광물의 일종인 rutile에서 유래 루틸의 결정 구조 Wine-red rutile crystals from Binn Valley, Switzerland



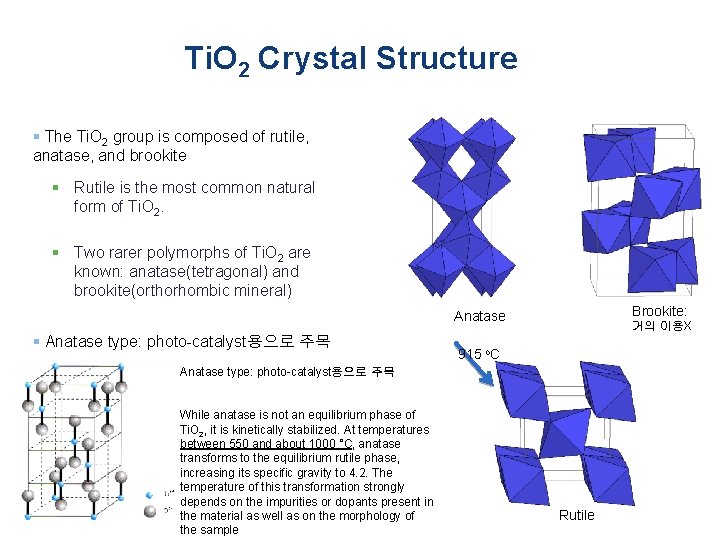
Ti. O 2 Crystal Structure § The Ti. O 2 group is composed of rutile, anatase, and brookite § Rutile is the most common natural form of Ti. O 2. § Two rarer polymorphs of Ti. O 2 are known: anatase(tetragonal) and brookite(orthorhombic mineral) Brookite: Anatase § Anatase type: photo-catalyst용으로 주목 거의 이용X 915 o. C Anatase type: photo-catalyst용으로 주목 While anatase is not an equilibrium phase of Ti. O 2, it is kinetically stabilized. At temperatures between 550 and about 1000 °C, anatase transforms to the equilibrium rutile phase, increasing its specific gravity to 4. 2. The temperature of this transformation strongly depends on the impurities or dopants present in the material as well as on the morphology of the sample Rutile

Chap. 2 2. 6 Diamond Type Crystal Structure

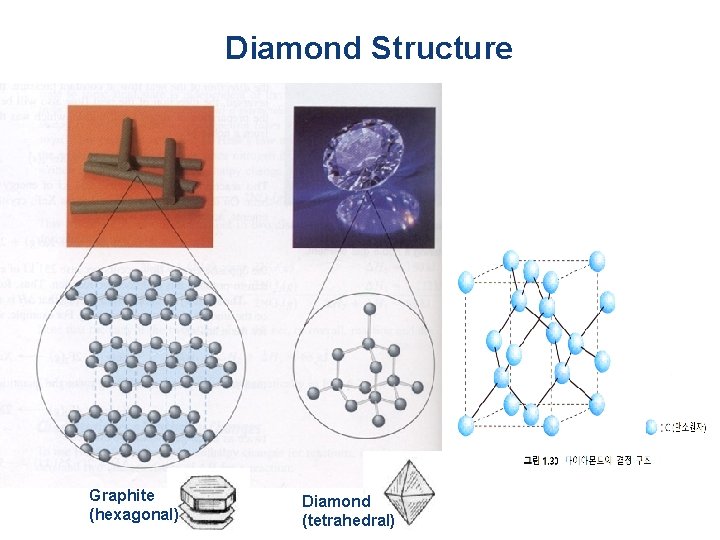
Diamond Structure Graphite (hexagonal) Diamond (tetrahedral)
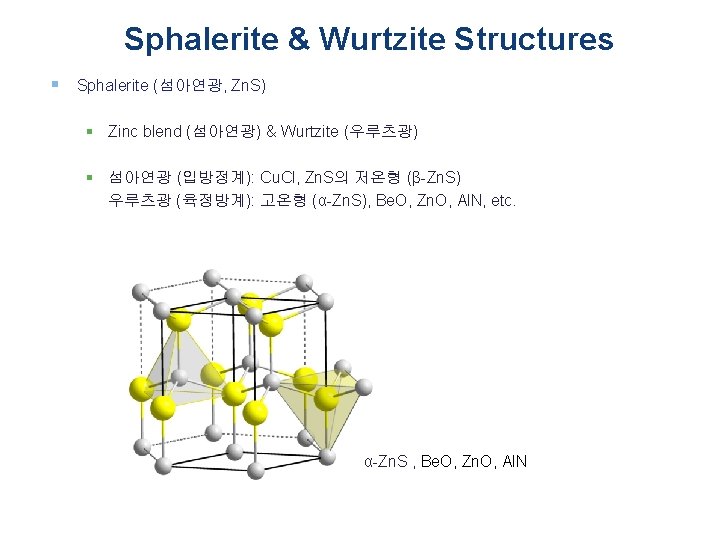
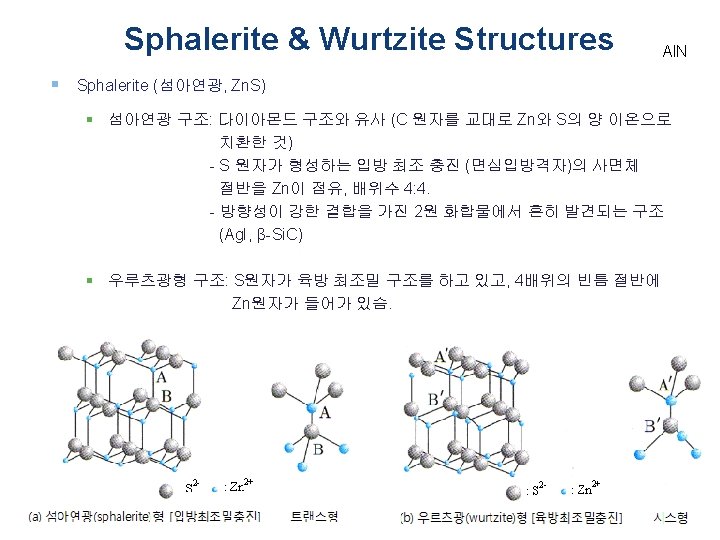
Sphalerite & Wurtzite Structures § Sphalerite (섬아연광, Zn. S) § Zinc blend (섬아연광) & Wurtzite (우루츠광) § 섬아연광 (입방정계): Cu. Cl, Zn. S의 저온형 (β-Zn. S) 우루츠광 (육정방계): 고온형 (α-Zn. S), Be. O, Zn. O, Al. N, etc. α-Zn. S , Be. O, Zn. O, Al. N
Chap. 2 2. 7 Layer Structure


Layer Structure § Cd. I 2(Cadmium iodide) structure I- Cd 2+
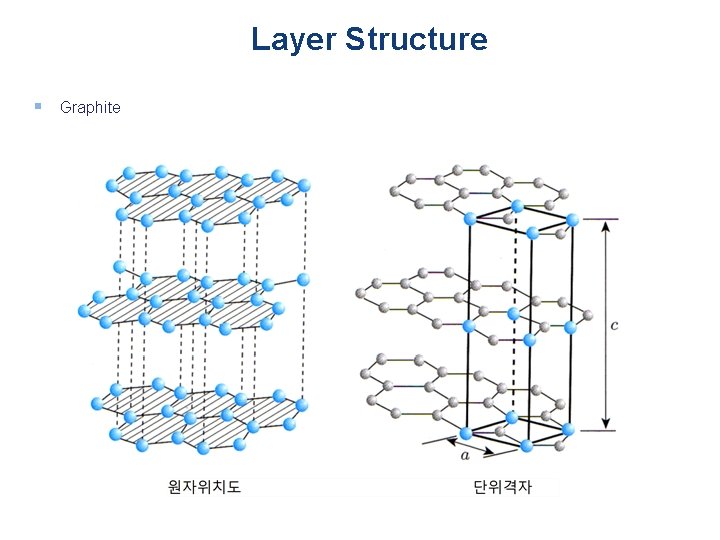
Layer Structure § Graphite
- Slides: 54