Inducing Apoptosis in Cancer Apoptosis is an Essential













- Slides: 28

Inducing Apoptosis in Cancer

Apoptosis is an Essential Process · Apoptosis (programmed cell death) plays an important role in normal development and homeostasis · Apoptosis is activated through two principal signaling pathways: intrinsic and extrinsic · Cancer is often initiated by DNA damage · Normal cells undergo apoptosis in response to stress-inducing events in the cell, such as DNA damage · Dysregulation of apoptosis is critical for cancer development and tumor cell survival 3

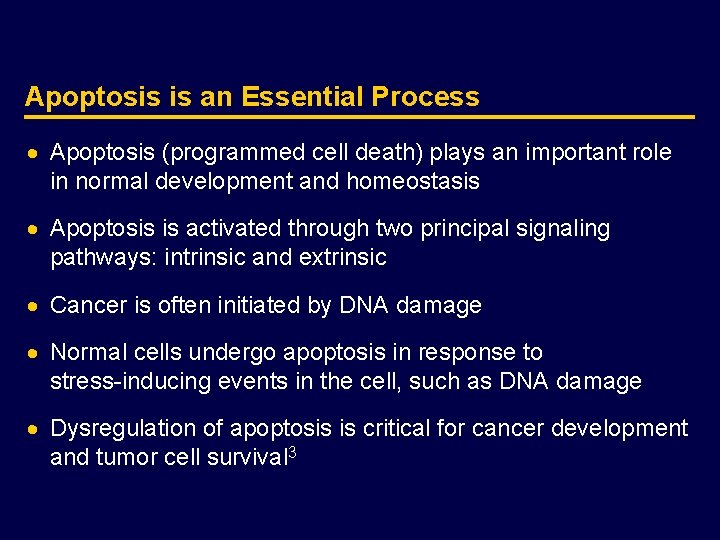
Overview of the Two Major Apoptosis Pathways Pro-apoptotic ligand Cell-extrinsic pathway Pro-apoptotic receptor Caspases BAX Mitochondria BCL 2 PUMA Apoptosis Stress Cell-intrinsic pathway Adapted from Ashkenazi A. Nat Rev Cancer 2002; 2: 420– 430. BAX, BCL 2 -associated protein; BCL 2, B-cell chronic lymphocytic leukemia/lymphoma 2; PUMA, p 53 -upregulated modulator of

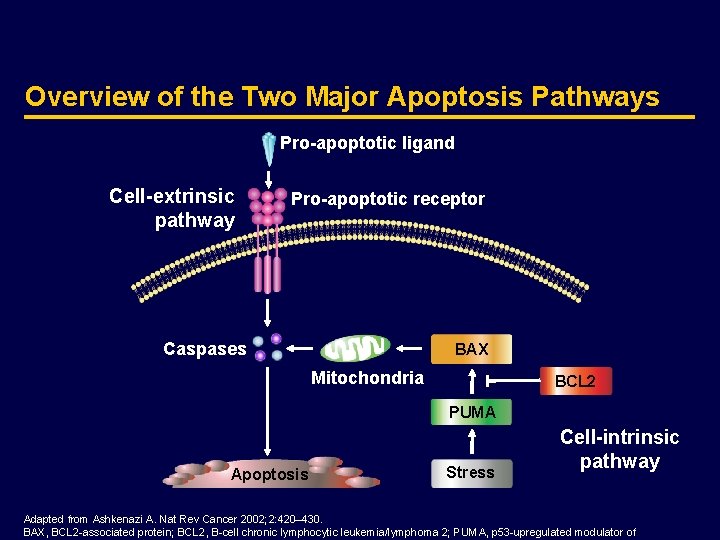
Apoptosis is Carried out by Caspases Pro-apoptotic stimulus Initiator caspases Effector caspases Apoptosis Adapted from Thornberry NA, Lazebnik Y. Science 1998; 281: 1312– 1316. Caspase, cysteine aspartase. Caspase cascade

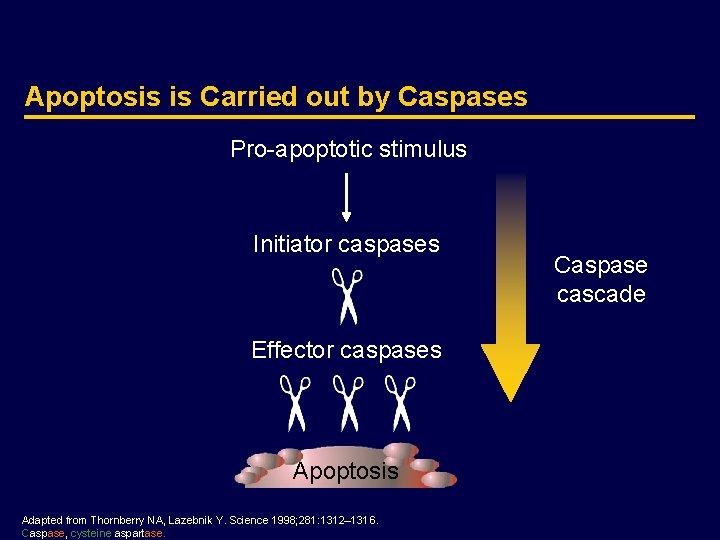
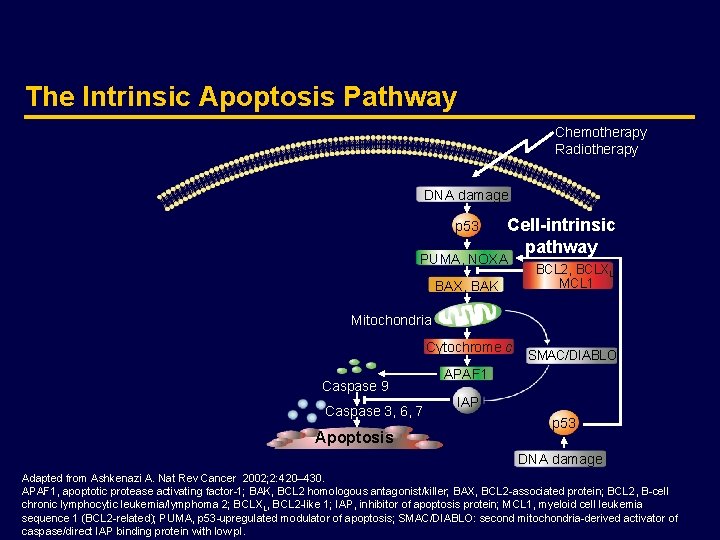
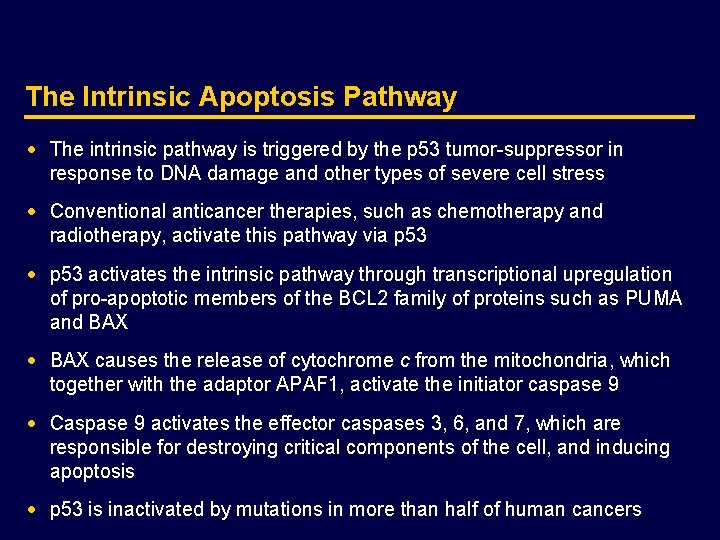
The Intrinsic Apoptosis Pathway Chemotherapy Radiotherapy DNA damage p 53 Cell-intrinsic pathway PUMA, NOXA BAX, BAK BCL 2, BCLXL, MCL 1 Mitochondria Cytochrome c Caspase 9 Caspase 3, 6, 7 Apoptosis SMAC/DIABLO APAF 1 IAP p 53 DNA damage Adapted from Ashkenazi A. Nat Rev Cancer 2002; 2: 420– 430. APAF 1, apoptotic protease activating factor-1; BAK, BCL 2 homologous antagonist/killer; BAX, BCL 2 -associated protein; BCL 2, B-cell chronic lymphocytic leukemia/lymphoma 2; BCLXL, BCL 2 -like 1; IAP, inhibitor of apoptosis protein; MCL 1, myeloid cell leukemia sequence 1 (BCL 2 -related); PUMA, p 53 -upregulated modulator of apoptosis; SMAC/DIABLO: second mitochondria-derived activator of caspase/direct IAP binding protein with low p. I.

The Intrinsic Apoptosis Pathway · The intrinsic pathway is triggered by the p 53 tumor-suppressor in response to DNA damage and other types of severe cell stress · Conventional anticancer therapies, such as chemotherapy and radiotherapy, activate this pathway via p 53 · p 53 activates the intrinsic pathway through transcriptional upregulation of pro-apoptotic members of the BCL 2 family of proteins such as PUMA and BAX · BAX causes the release of cytochrome c from the mitochondria, which together with the adaptor APAF 1, activate the initiator caspase 9 · Caspase 9 activates the effector caspases 3, 6, and 7, which are responsible for destroying critical components of the cell, and inducing apoptosis · p 53 is inactivated by mutations in more than half of human cancers

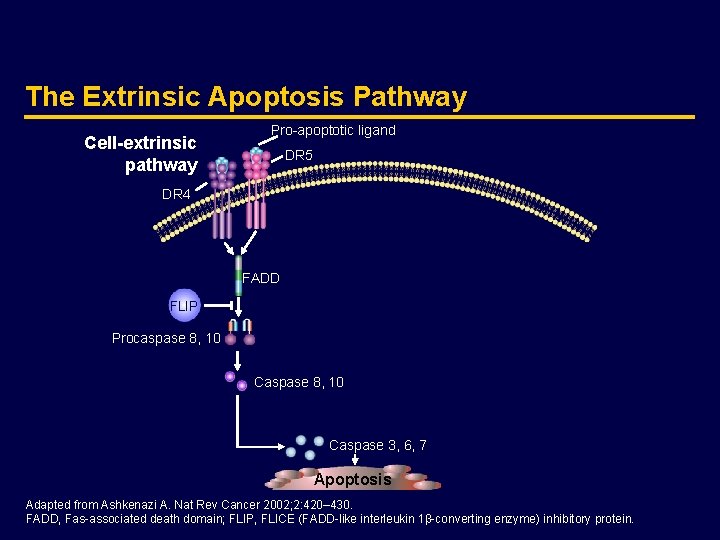
The Extrinsic Apoptosis Pathway Cell-extrinsic pathway Pro-apoptotic ligand DR 5 DR 4 FADD FLIP Procaspase 8, 10 Caspase 3, 6, 7 Apoptosis Adapted from Ashkenazi A. Nat Rev Cancer 2002; 2: 420– 430. FADD, Fas-associated death domain; FLIP, FLICE (FADD-like interleukin 1β-converting enzyme) inhibitory protein.

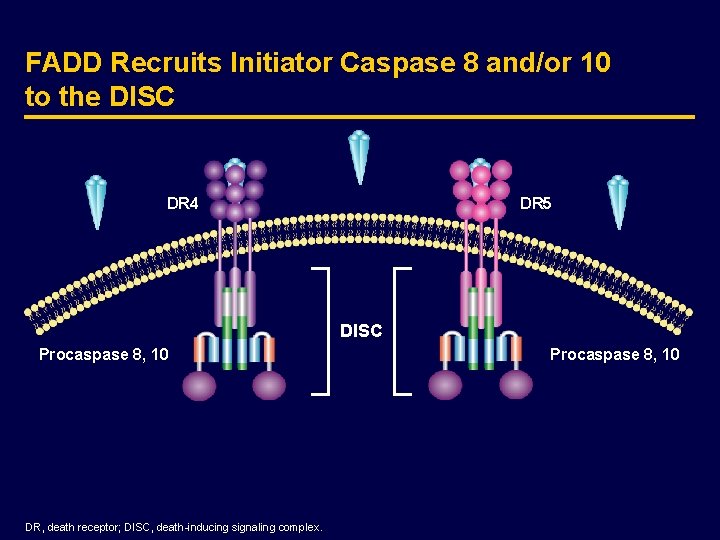
The Extrinsic Apoptosis Pathway · The extrinsic pathway triggers apoptosis in response to the activation of pro-apoptotic receptors, such as DR 4 and DR 5, by specific pro-apoptotic ligands, such as Apo 2 L/TRAIL · This pathway stimulates apoptosis independently of p 53 · Ligand-induced activation of DR 4 and DR 5 leads to the rapid assembly of the death-inducing signaling complex (DISC) and the recruitment of initiator caspases 8 and 10 through the adaptor Fas-associated death domain (FADD) · Caspases 8 and 10 activate effector caspases 3, 6, and 7, leading to apoptosis

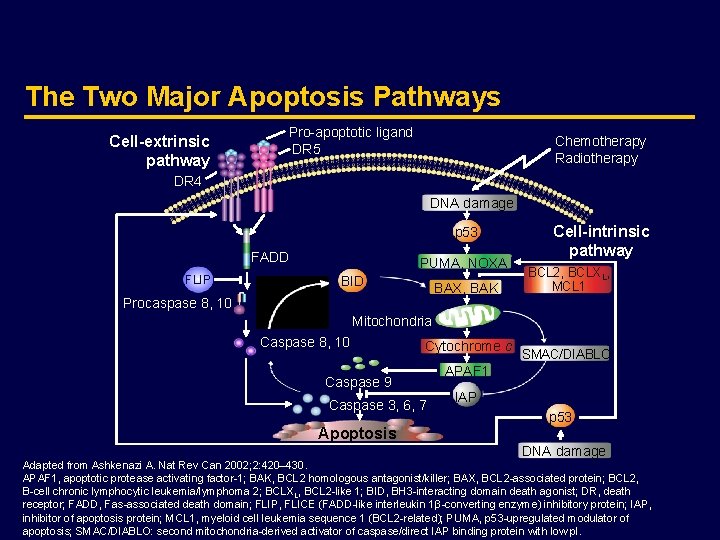
The Two Major Apoptosis Pathways Cell-extrinsic pathway Pro-apoptotic ligand DR 5 Chemotherapy Radiotherapy DR 4 DNA damage p 53 FADD FLIP PUMA, NOXA BID BAX, BAK Cell-intrinsic pathway BCL 2, BCLXL, MCL 1 Procaspase 8, 10 Mitochondria Caspase 8, 10 Cytochrome c Caspase 9 Caspase 3, 6, 7 Apoptosis SMAC/DIABLO APAF 1 IAP p 53 DNA damage Adapted from Ashkenazi A. Nat Rev Can 2002; 2: 420– 430. APAF 1, apoptotic protease activating factor-1; BAK, BCL 2 homologous antagonist/killer; BAX, BCL 2 -associated protein; BCL 2, B-cell chronic lymphocytic leukemia/lymphoma 2; BCLXL, BCL 2 -like 1; BID, BH 3 -interacting domain death agonist; DR, death receptor; FADD, Fas-associated death domain; FLIP, FLICE (FADD-like interleukin 1β-converting enzyme) inhibitory protein; IAP, inhibitor of apoptosis protein; MCL 1, myeloid cell leukemia sequence 1 (BCL 2 -related); PUMA, p 53 -upregulated modulator of apoptosis; SMAC/DIABLO: second mitochondria-derived activator of caspase/direct IAP binding protein with low p. I.

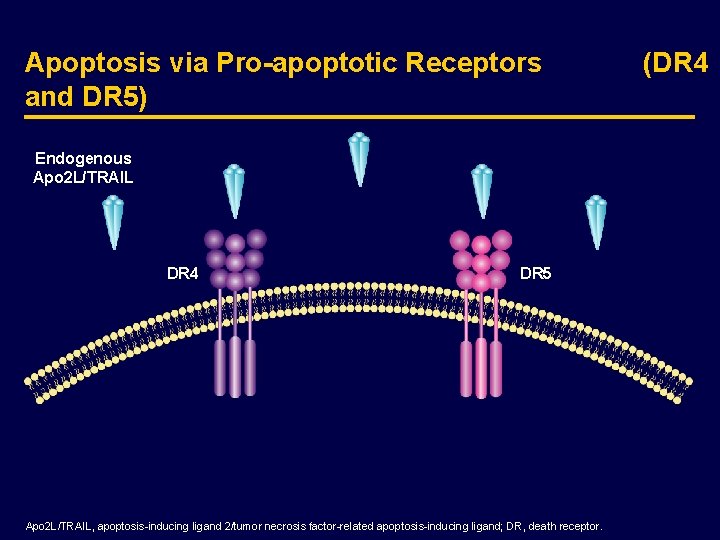
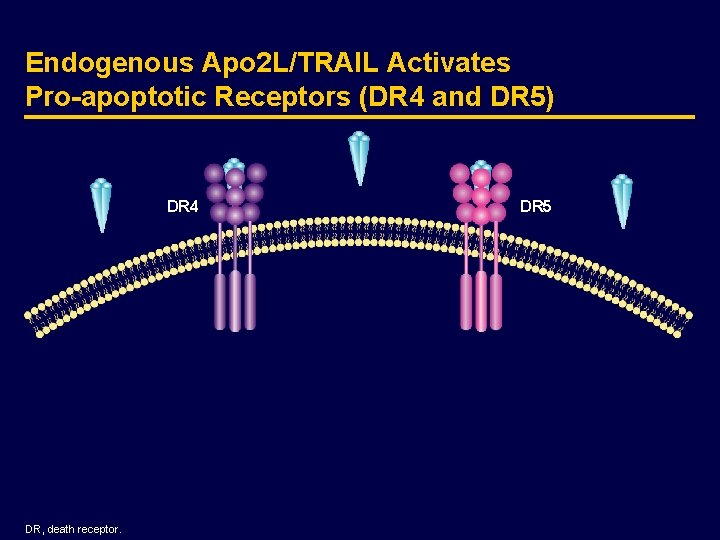
Apoptosis via Pro-apoptotic Receptors and DR 5) Endogenous Apo 2 L/TRAIL DR 4 DR 5 Apo 2 L/TRAIL, apoptosis-inducing ligand 2/tumor necrosis factor-related apoptosis-inducing ligand; DR, death receptor. (DR 4

Endogenous Apo 2 L/TRAIL Activates Pro-apoptotic Receptors (DR 4 and DR 5) DR 4 DR, death receptor. DR 5

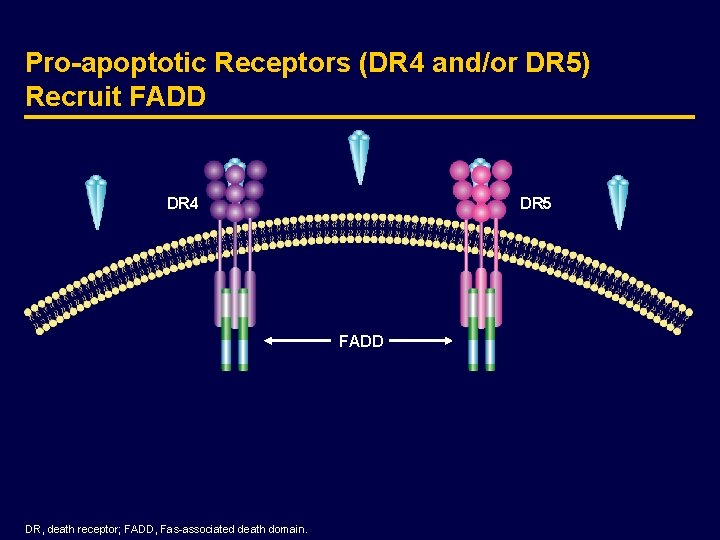
Pro-apoptotic Receptors (DR 4 and/or DR 5) Recruit FADD DR 4 DR 5 FADD DR, death receptor; FADD, Fas-associated death domain.

FADD Recruits Initiator Caspase 8 and/or 10 to the DISC DR 4 DR 5 DISC Procaspase 8, 10 DR, death receptor; DISC, death-inducing signaling complex. Procaspase 8, 10

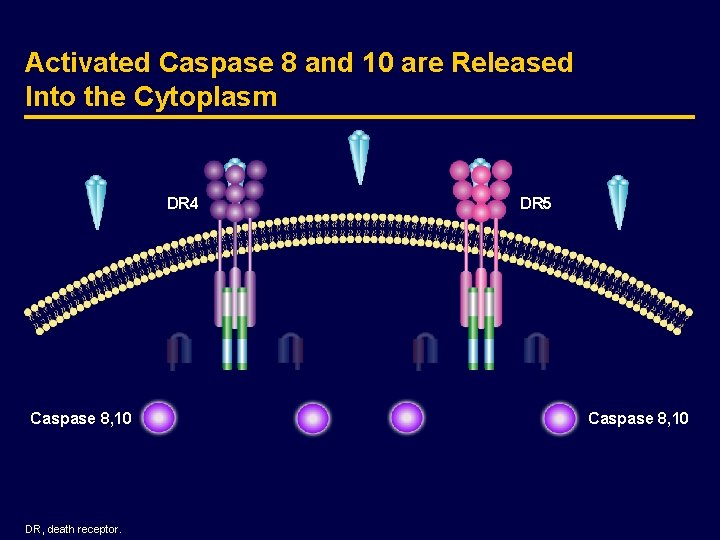
Activated Caspase 8 and 10 are Released Into the Cytoplasm DR 4 Caspase 8, 10 DR, death receptor. DR 5 Caspase 8, 10

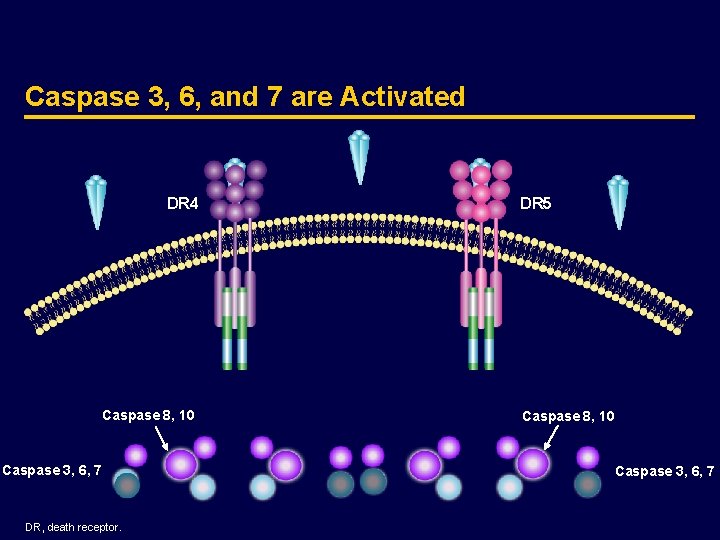
Caspase 3, 6, and 7 are Activated DR 4 Caspase 8, 10 Caspase 3, 6, 7 DR, death receptor. DR 5 Caspase 8, 10 Caspase 3, 6, 7

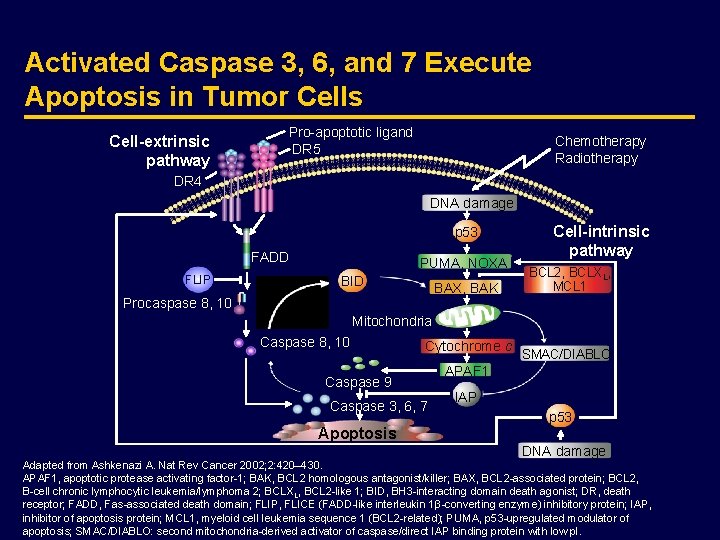
Activated Caspase 3, 6, and 7 Execute Apoptosis in Tumor Cells Cell-extrinsic pathway Pro-apoptotic ligand DR 5 Chemotherapy Radiotherapy DR 4 DNA damage p 53 FADD FLIP PUMA, NOXA BID BAX, BAK Cell-intrinsic pathway BCL 2, BCLXL, MCL 1 Procaspase 8, 10 Mitochondria Caspase 8, 10 Cytochrome c Caspase 9 Caspase 3, 6, 7 Apoptosis SMAC/DIABLO APAF 1 IAP p 53 DNA damage Adapted from Ashkenazi A. Nat Rev Cancer 2002; 2: 420– 430. APAF 1, apoptotic protease activating factor-1; BAK, BCL 2 homologous antagonist/killer; BAX, BCL 2 -associated protein; BCL 2, B-cell chronic lymphocytic leukemia/lymphoma 2; BCLXL, BCL 2 -like 1; BID, BH 3 -interacting domain death agonist; DR, death receptor; FADD, Fas-associated death domain; FLIP, FLICE (FADD-like interleukin 1β-converting enzyme) inhibitory protein; IAP, inhibitor of apoptosis protein; MCL 1, myeloid cell leukemia sequence 1 (BCL 2 -related); PUMA, p 53 -upregulated modulator of apoptosis; SMAC/DIABLO: second mitochondria-derived activator of caspase/direct IAP binding protein with low p. I.

References 1. Ashkenazi A. Targeting death and decoy receptors of the tumor-necrosis factor superfamily. Nat Rev Cancer 2002; 2: 420– 430. 2. Ghobrial IM, Witzig TE, Adjei AA. Targeting apoptosis pathways in cancer therapy. CA Cancer J Clin 2005; 55: 178– 194. 3. Hanahan D, Weinberg RA. The hallmarks of cancer. Cell 2000; 100: 57– 70. 4. Thornberry NA, Lazebnik Y. Caspases: Enemies within. Science 1998; 281: 1312– 1316. 5. Ashkenazi A, Pai RC, Fong S, et al. Safety and antitumor activity of recombinant soluble Apo 2 ligand. J Clin Invest 1999; 104: 155– 162. 6. Lowe SW, Bodis S, Mc. Clatchey A, et al. p 53 status and the efficacy of cancer therapy in vivo. Science 1994; 266: 807– 810. 7. Lee JM, Bernstein A. Apoptosis, cancer, and the p 53 tumor suppressor gene. Cancer Metastasis Rev 1995; 14: 149– 161. 8. Igney FH, Krammer PH. Death and anti-death: tumor resistance to apoptosis. Nat Rev Cancer 2002; 2: 277– 288.

References (cont’d) 9. Pan G, O’Rourke K, Chinnaiyan AM, et al. The receptor for the cytotoxic ligand TRAIL. Science 1997; 276: 111– 113. 10. Sheridan JP, Marsters SA, Pitti RM, et al. Control of TRAIL-induced apoptosis by the family of signaling and decoy receptors. Science 1997; 277: 818– 821. 11. Pitti RM, Marsters SA, Ruppert S, et al. Induction of apoptosis by Apo-2 ligand, a new member of the tumor necrosis factor cytokine family. J Biol Chem 1996; 271: 12687– 12690. 12. Wiley SR, Schooley K, Smolak PJ, et al. Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity 1995; 3: 673– 682. 13. Gazitt Y. TRAIL is a potent inhibitor of apoptosis in myeloma cells derived from multiple myeloma patients and is not cytotoxic to hematopoietic stem cells. Leukemia 1999; 13: 1817– 1824. 14. Pollack IF, Erff M, Ashkenazi A. Direct stimulation of apoptotic signaling by soluble Apo 2 L/tumor necrosis factor-related apoptosis-inducing ligand leads to selective killing of glioma cells. Clin Cancer Res 2001; 7: 1362– 1369.

References (cont’d) 15. Qin J-Z, Chaturvedi V, Bonish B, Nickoloff BJ. Avoiding premature apoptosis of normal epidermal cells. Nat Med 2001; 7: 385– 386. 16. Kischkel FC, Lawrence DA, Chuntharapai A, et al. Apo 2 L/TRAIL-dependent recruitment of endogenous FADD and caspase-8 to death receptors 4 and 5. Immunity 2000; 12: 611 – 620. 17. Chinnaiyan AM, O’Rourke K, Tewari M, Dixit VM. FADD, a novel death domaincontaining protein, interacts with the death domain of Fas and initiates apoptosis. Cell 1995; 81: 505– 512. 18. Kischkel FC, Lawrence DA, Tinel A, et al. Death receptor recruitment of endogenous caspase-10 and apoptosis initiation in the absence of caspase-8. J Biol Chem 2001; 276: 46639– 46646. 19. Griffith TS, Lynch DH. TRAIL: a molecule with multiple receptors and control mechanisms. Curr Opin Immunol 1998; 10: 559– 563. 20. Pukac L, Kanakaraj P, Humphreys R, et al. HGS-ETR 1, a fully human TRAIL-receptor 1 monoclonal antibody, induces cell death in multiple tumor types in vitro and in vivo. Br J Cancer 2005; 92: 1430– 1441.

Apoptosis For every cell, there is a time to live and a time to die. There are two ways in which cells die: · · They are killed by injurious agents. They are induced to commit suicide. Death by injury Cells that are damaged by injury, such as by · mechanical damage · exposure to toxic chemicals

Undergo a characteristic series of changes: · · They (and their organelles like mitochondria) swell (because the ability of the plasma membrane to control the passage of ions and water is disrupted). The cell contents leak out, leading to inflammation of surrounding tissues.

Death by suicide Cells that are induced to commit suicide: · shrink; · develop bubble-like blebs on their surface; · have the chromatin (DNA and protein) in their nucleus degraded; · have their mitochondria break down with the release of cytochrome C; · break into small, membrane-wrapped, fragments. · The phospholipid phosphatidylserine, which is normally hidden within the plasma membrane, is exposed on the surface. · This is bound by receptors on phagocytic cells like macrophages and dentritic cells which then engulf the cell fragments. · The phagocytic cells secrete cytokines that inhibit inflammation (e. g. , IL 10 and TGF- β) The pattern of events in death by suicide is so orderly that the process is often called programmed cell death or PCD. The cellular machinery of programmed cell death turns out to be as intrinsic to the cell as, say, mitosis.

Why should a cell commit suicide? There are two different reasons. 1. Programmed cell death is as needed for proper development as mitosis is. · Examples: · The resorption of the tadpole tail at the time of its metamorphosis into a frog occurs by apoptosis. · The formation of the fingers and toes of the fetus requires the removal, by apoptosis, of the tissue between them. · The sloughing off of the inner lining of the uterus (the endometrium) at the start of (menstruation) occurs by apoptosis. · The formation of the proper connections (synapses) between neurons in the brain requires that surplus cells be eliminated by apoptosis

Why should a cell commit suicide? There are two different reasons. 2. Programmed cell death is needed to destroy cells that represent a threat to the integrity of the organism. Cells infected with viruses · One of the methods by which cytotoxic Lymphocytes (CTLs) kill virusinfected cells is by inducing apoptosis. (And some viruses mount countermeasures to thwart it). Cells of the immune system · As cell-mediated immune responses wane, the effector cells must be removed to prevent them from attacking body constituents. CTLs induce apoptosis in each other and even in themselves. Defects in the apoptotic machinery is associated with autoimmune responses such as lupus erythematosus and rheumatoid arthritis. Cells with DNA damage · Damage to its genome can cause a cell · to disrupt proper embryonic development leading to birth defects · to become cancerous.

Why should a cell commit suicide? There are two different reasons. Cells respond to DNA damage by increasing their production of p 53 oncogene is a potent inducer of apoptosis. Is it any wonder that mutations in the p 53 gene, producing a defective protein, are so often found in cancer cells (that represent a lethal threat to the organism if permitted to live)? Cancer cells · Radiation and Chemotherapy used in cancer therapy induce apoptosis in some types of cancer cells.

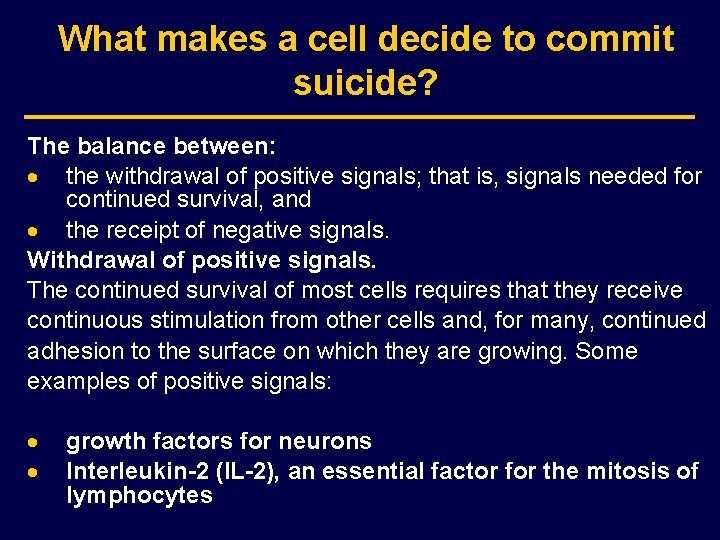
What makes a cell decide to commit suicide? The balance between: · the withdrawal of positive signals; that is, signals needed for continued survival, and · the receipt of negative signals. Withdrawal of positive signals. The continued survival of most cells requires that they receive continuous stimulation from other cells and, for many, continued adhesion to the surface on which they are growing. Some examples of positive signals: · · growth factors for neurons Interleukin-2 (IL-2), an essential factor for the mitosis of lymphocytes

What makes a cell decide to commit suicide? Receipt of negative signals · · increased levels of oxidants within the cell damage to DNA by these oxidants or other agents like – ultraviolet light – x-rays – Chemotherapy drugs accumulation of proteins that failed to fold properly into their proper tertiary structure molecules that bind to specific receptors on the cell surface and signal the cell to begin the apoptosis program. These death activators include: – Tumor necrosis factor-alpha (TNF-α ) that binds to the TNF receptor; – Lymphotoxin (also known as TNF-β ) that also binds to the TNF receptor; – Fas ligand (Fas. L), a molecule that binds to a cell-surface receptor named Fas (also called CD 95).

Apoptosis in Plants · · · Plant, too, can turn on a system of programmed cell death; for example, in an attempt to halt the spread of virus infection. The mechanism differs from that in animals although it, too, involves a protease that — like caspases — cleaves other proteins at Asp (and. Asn) residues. Activation of this enzyme destroys the central vacuole, which is followed by disintegration of the rest of the cell.