Indian Medical Device Industry Related Regulations Rajiv Nath














- Slides: 23

Indian Medical Device Industry & Related Regulations Rajiv Nath , President , AISNMA Forum Coordinator , AIMED

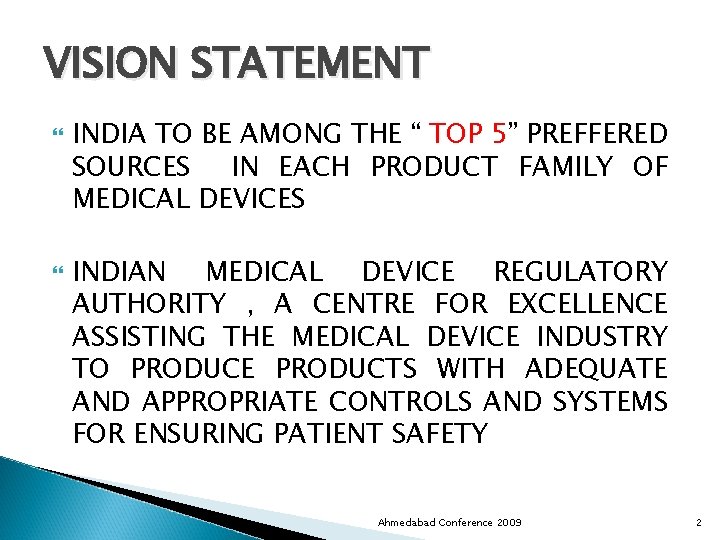
VISION STATEMENT INDIA TO BE AMONG THE “ TOP 5” PREFFERED SOURCES IN EACH PRODUCT FAMILY OF MEDICAL DEVICES INDIAN MEDICAL DEVICE REGULATORY AUTHORITY , A CENTRE FOR EXCELLENCE ASSISTING THE MEDICAL DEVICE INDUSTRY TO PRODUCE PRODUCTS WITH ADEQUATE AND APPROPRIATE CONTROLS AND SYSTEMS FOR ENSURING PATIENT SAFETY Ahmedabad Conference 2009 2

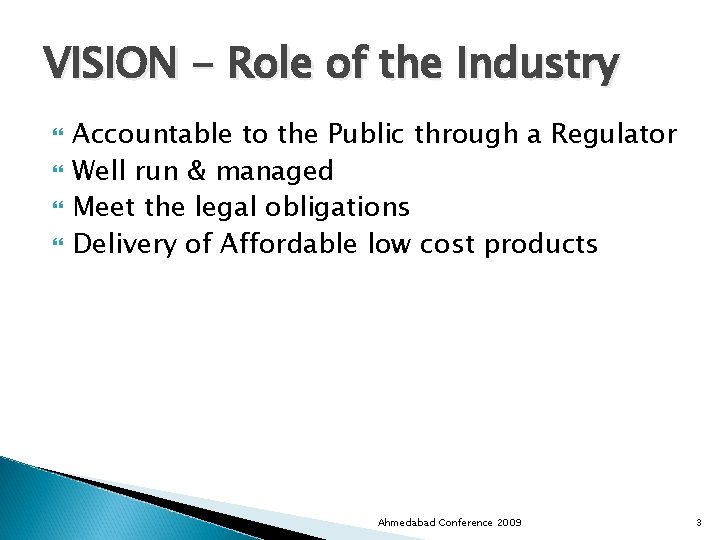
VISION - Role of the Industry Accountable to the Public through a Regulator Well run & managed Meet the legal obligations Delivery of Affordable low cost products Ahmedabad Conference 2009 3

VISION - Role of the Regulator § Work with Industry in transparent manner Supervise Directly / Through Accredited agencies Aim to promote public trust in Indian Industry Provide Guidance & Advice to meet legal obligations safety of consumers Facilitate the Growth of Industry § Facilitate Introduction of Innovative & Emerging Technologies 4

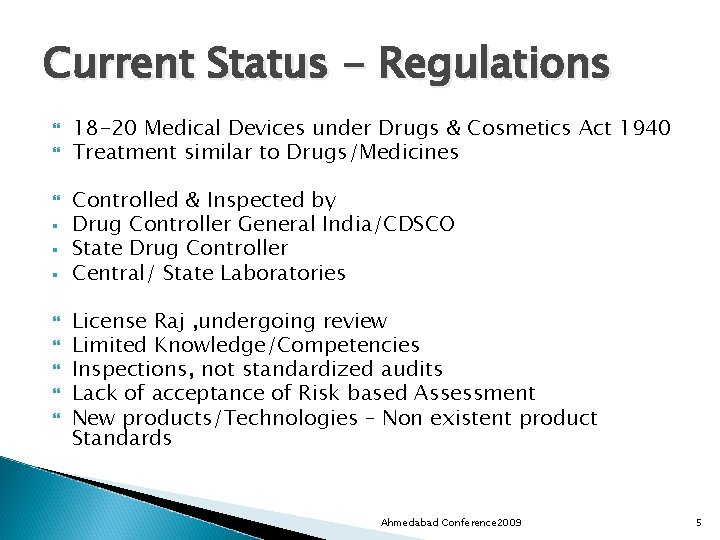
Current Status - Regulations § § § 18 -20 Medical Devices under Drugs & Cosmetics Act 1940 Treatment similar to Drugs/Medicines Controlled & Inspected by Drug Controller General India/CDSCO State Drug Controller Central/ State Laboratories License Raj , undergoing review Limited Knowledge/Competencies Inspections, not standardized audits Lack of acceptance of Risk based Assessment New products/Technologies – Non existent product Standards Ahmedabad Conference 2009 5

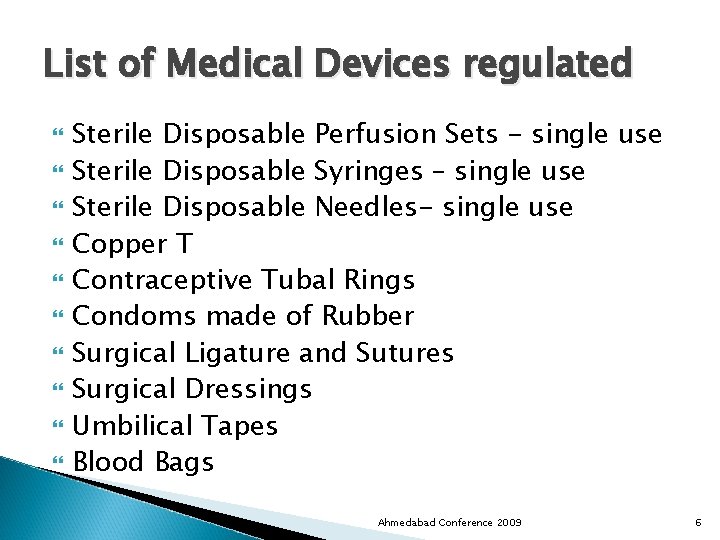
List of Medical Devices regulated Sterile Disposable Perfusion Sets - single use Sterile Disposable Syringes – single use Sterile Disposable Needles- single use Copper T Contraceptive Tubal Rings Condoms made of Rubber Surgical Ligature and Sutures Surgical Dressings Umbilical Tapes Blood Bags Ahmedabad Conference 2009 6

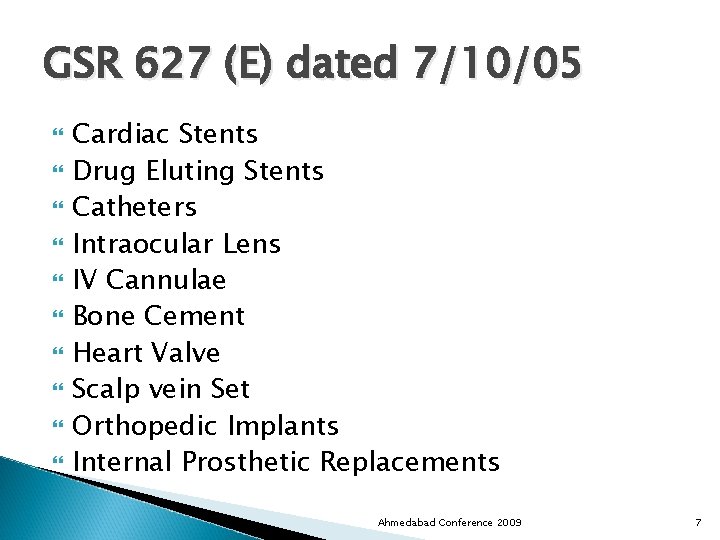
GSR 627 (E) dated 7/10/05 Cardiac Stents Drug Eluting Stents Catheters Intraocular Lens IV Cannulae Bone Cement Heart Valve Scalp vein Set Orthopedic Implants Internal Prosthetic Replacements Ahmedabad Conference 2009 7

GHTF-Global Harmonization Task Force Founded in 1992 by Canada, EU, Japan , USA , Australia Informal Grouping of medical device regulators & industry To encourage convergence in regulatory practices related to ensuring the safety, effectiveness/performance and quality of medical devices , promoting technological innovation and facilitating international trade By publication of harmonized guidance documents on basic regulatory practices Ahmedabad Conference 2009 8

AHWP-Asian Harmonization Working Party Formed in 1996 by an informal group of experts from regulatory authorities , CAB s & industry To forge a common direction for harmonization of medical device regulation in Asia Encourage understanding on benefits of harmonization and facilitate a linkage with the GHTF Provide a forum for discussion & training Ahmedabad Conference 2009 9

AIMED Association of Indian Medical Device Industry IMDRRG Indian Medical Devices Regulatory Review Group

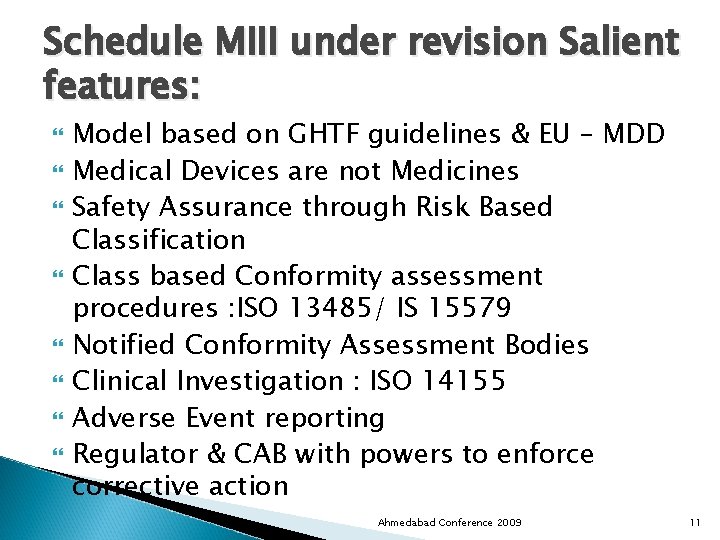
Schedule MIII under revision Salient features: Model based on GHTF guidelines & EU – MDD Medical Devices are not Medicines Safety Assurance through Risk Based Classification Class based Conformity assessment procedures : ISO 13485/ IS 15579 Notified Conformity Assessment Bodies Clinical Investigation : ISO 14155 Adverse Event reporting Regulator & CAB with powers to enforce corrective action Ahmedabad Conference 2009 11

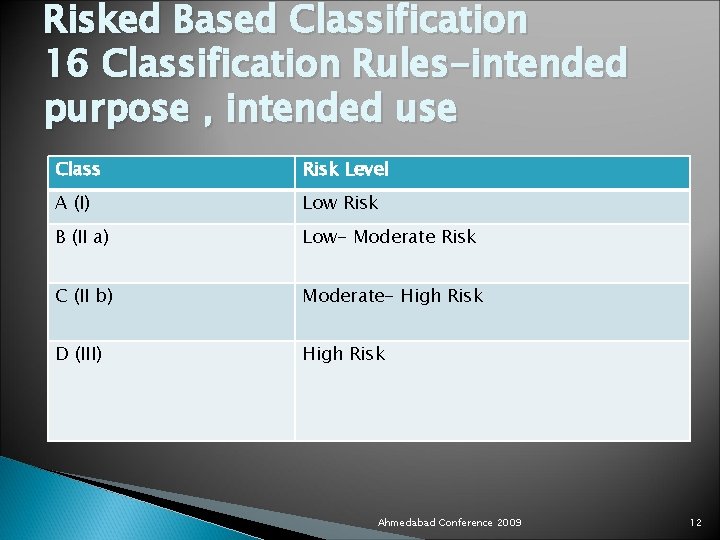
Risked Based Classification 16 Classification Rules-intended purpose , intended use Class Risk Level A (I) Low Risk B (II a) Low- Moderate Risk C (II b) Moderate- High Risk D (III) High Risk Ahmedabad Conference 2009 12

Risk based Regulation Degree Class A - Manufacturer will register with CLAA - Manufacturing license not required Class B – Manufacturers Quality Management System to be assessed and certified by a notified body - Manufacturer will be registered with the CLAA on the basis of certificate from notified body - Manufacturing License not required Class C – Certification by a notified body is required with regard to the design & manufacture of the device -manufacturer to apply for manufacturing license to CLAA with supporting documents wrt safety & efficacy of device -Manufacturing license to be issued by CLAA on the basis of above documents and certificate issued by CAB Class D – As ‘C’ above+ Factory will be jointly inspected by CLAA & State licensing authority , MFG LIC based on report + Certificate from Notified body 13

ISO 13485 Quality Management System Manufacturing Facility Compliance Process Approach – Model , 8 Broad Sections Activities – Quality Plan , Quality Objectives, Internal Audits , Corrective & Preventive Action, independent external audits & Tests Enables Response to External issuescustomer complaints/ feedback , regulatory or Internal issues-facilities, process up gradation or training and competency of personnel Ahmedabad Conference 2009 14

Risk Management ISO 14971 -Analysing, Evaluating & Controlling Risks Develop risk management plan Failure Mode & Effects Analysis (FMEA) Fault Tree Analysis (FTA) Identification of Hazard Preliminary Risk Assessment Risk Mitigation Residual Risk evaluation Ahmedabad Conference 2009 15

AIMED Meeting with DGCI 1) Free Sales Certificate Issue - Informal Registration Information -ISO 13485/CE certification -Prior History of Mfg/ Exports -Informal cursory inspection to validate -Free Sales Certificate Issuance -Undertaking to Fulfill Licensing Requirements 2) ISO 13485 QMS in transition period instead Schedule M/MIII Ahmedabad Conference 2009 16

Transitional Guidelines Grant of license, registration & fees on basis of product family Brand Permission to be changed to Additional Brand information if identical product Parametric Release if Process validated for EO sterilization & Pharmacopeia Review Guideline for minor/major NCR for substandard devices & BIS/ISO/Co. standard Training of Regulators with Industry Inputs Standardize Formats –Non Conviction, Market Standing & Performance Certificates Ahmedabad Conference 2009 17

Current Status - Industry Majority Exporting units comply with ISO 13485 and Certificate/Registration with GHTF countries Moving up the Value Chain Moving up the Quality & Technology Ladder “Top 5” Preferred Source Status achieved in: 1) 2) 3) 4) 5) 6) Syringes Needles I. V. Cannulas /Catheters Contraceptives Surgical Blades Gloves 7) Intraocular Lens Ahmedabad Conference 2009 18

Path for the Industry § § § Upgrade: manufacturing & testing facilities management competencies quality management systems Get ISO 13485 certification from internationally accredited organizations Get registration and certification from a GHTF member country Self regulation/ Continue Dialog with MOH to assist in building regulatory framework Ahmedabad Conference 2009 19

Path for the Regulator Continue the Improved transparency & dialog Continue to Involve Industry in creating a mutually acceptable Regulatory Framework & Infrastructure. Ensure timely response to guidance/ advice sought on clarifications of compliances Constitute expert committees on various aspects Define milestones for phased creation of a regulatory infrastructure & implementation Ahmedabad Conference 2009 20

If Things go wrong …. . Regulations to provide non ambiguous legal requirements & guidance on best practices Reliance on preventing problems Evaluation of Risk & Hazard by MDR for suitable corrective & preventive action (CAPA) Reasonable time for addressing issues and implementing CAPA Regulator to have powers for putting things right Minimal/No reliance of judiciary and legal criminal action on registered units Reliance of Judiciary/ Police and criminal action only on non registered units. Ahmedabad Conference 2009 21

Pitfalls & Avoidance Lack of trust and dialog Lack of time bound response to queries Reliance on Tests , inspections, arbitrary controls Draconian punitive action Unreasonable expectations Lack of clarity of superseded rules/requirements and standards Conformity assessment organizations acting as consultants / trainers to same assesses Ahmedabad Conference 2009 22

Lets Work in Partnership to achieve The Millennium Development Goals Thank You! Ahmedabad Conference 2009 23
 Yatindra nath singh
Yatindra nath singh Shamindra nath sanyal
Shamindra nath sanyal Properties of bose-einstein condensate
Properties of bose-einstein condensate Fag bearings india limited
Fag bearings india limited Dr reshmi nath
Dr reshmi nath Dr umanath ias
Dr umanath ias Arindam nath
Arindam nath Pupil in death
Pupil in death Internal input devices
Internal input devices Rajiv gandhi groundwater raipur
Rajiv gandhi groundwater raipur True labour pains
True labour pains Ca rajiv singh
Ca rajiv singh Rajiv roy md
Rajiv roy md Rajiv vidya mission
Rajiv vidya mission Ca rajiv singh
Ca rajiv singh Gentoo graphical installer
Gentoo graphical installer The integration of eye, hand, and foot movements
The integration of eye, hand, and foot movements Health-related skills
Health-related skills Evolution of leasing in india
Evolution of leasing in india Indian plastic industry analysis
Indian plastic industry analysis Indian oil corporation retired employees
Indian oil corporation retired employees A tagout device is preferable to using a lockout device.
A tagout device is preferable to using a lockout device. Kelompok output
Kelompok output Gmdn udi
Gmdn udi