Incretinbased therapies Part I F Hosseinpanah Obesity Research

Incretin-based therapies Part I F. Hosseinpanah Obesity Research Center Research Institute for Endocrine sciences Shahid Beheshti University of Medical Sciences November 23, 2014
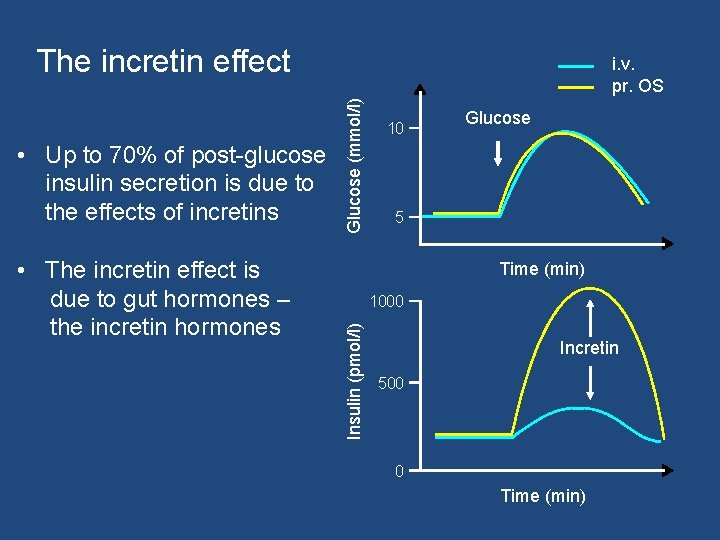
The incretin effect • The incretin effect is due to gut hormones – the incretin hormones Glucose (mmol/l) 10 Glucose 5 Time (min) 1000 Insulin (pmol/l) • Up to 70% of post-glucose insulin secretion is due to the effects of incretins i. v. pr. OS Incretin 500 0 Time (min)

GLP-1 and GIP Are the Two Major Incretins GLP-1 GIP • Potentiates glucose-induced insulin secretion • Upregulates insulin gene expression and all steps in insulin biosynthesis • Upregulates expresssion of other genes essential for β-cell function • Enhances β-cell proliferation and survival in animal models and isolated human islets Other effects • Suppresses hepatic glucose output by inhibiting glucagon secretion in a glucose-dependent manner • Inhibits gastric emptying and appetite; reduction of food intake and body weight • Potentiates glucose-induced insulin secretion • Upregulates insulin gene expression and all steps in insulin biosynthesis • Upregulates expresssion of other genes essential for β-cell function • Enhances β-cell proliferation and survival in islet cell lines • Does not inhibit glucagon secretion • Minimal effects on gastric emptying; no significant effects on appetite or body weight Holst et al, FEBS letts 1987; Ørskov et al Endocrinology 1988; Wettergren et al , Dig Dis Sci. 1993 ; Flint et al J Clin Invest 1998 ; Holst JJ, Physiol Rev 2007; Trümper A et al. Mol Endocrinol. 2001; 15: 1559– 1570; Trümper A et al. J Endocrinol. 2002; 174: 233– 246; Wideman RD et al. Horm Metab Res. 2004; 36: 782– 786.
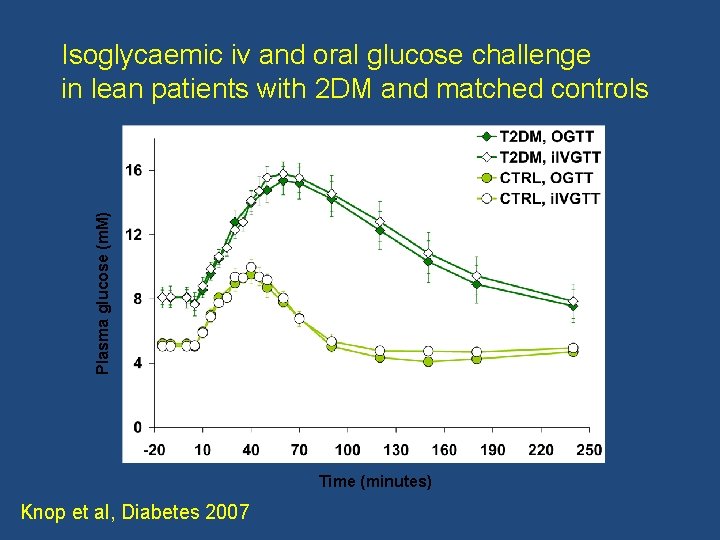
Plasma glucose (m. M) Isoglycaemic iv and oral glucose challenge in lean patients with 2 DM and matched controls Time (minutes) Knop et al, Diabetes 2007
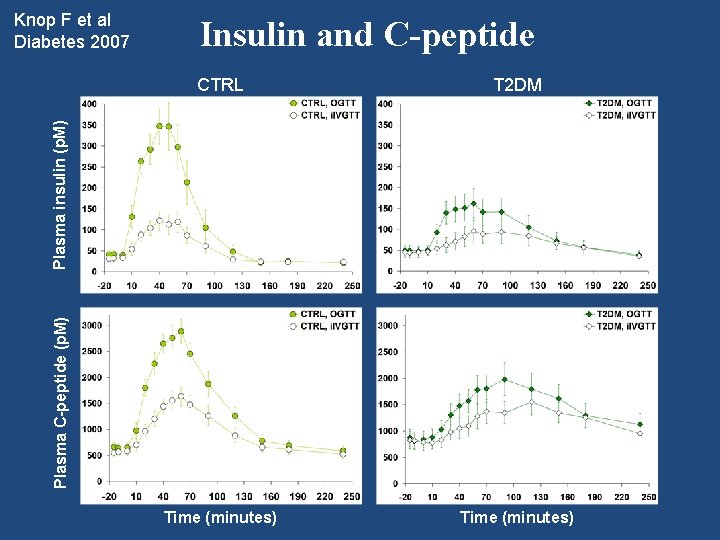
Insulin and C-peptide CTRL T 2 DM Time (minutes) Plasma C-peptide (p. M) Plasma insulin (p. M) Knop F et al Diabetes 2007
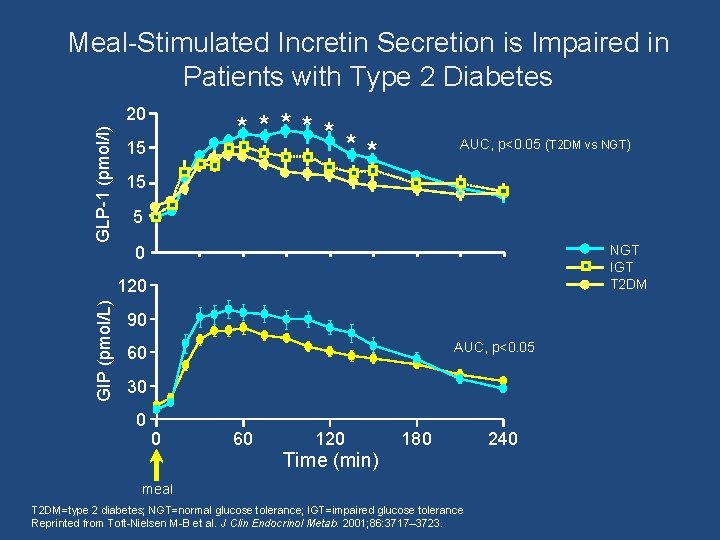
Meal-Stimulated Incretin Secretion is Impaired in Patients with Type 2 Diabetes GLP-1 (pmol/l) 20 ***** ** 15 AUC, p<0. 05 (T 2 DM vs NGT) 15 5 NGT IGT T 2 DM 0 GIP (pmol/L) 120 90 AUC, p<0. 05 60 30 0 0 60 120 180 Time (min) meal T 2 DM=type 2 diabetes; NGT=normal glucose tolerance; IGT=impaired glucose tolerance Reprinted from Toft-Nielsen M-B et al. J Clin Endocrinol Metab. 2001; 86: 3717– 3723. 240
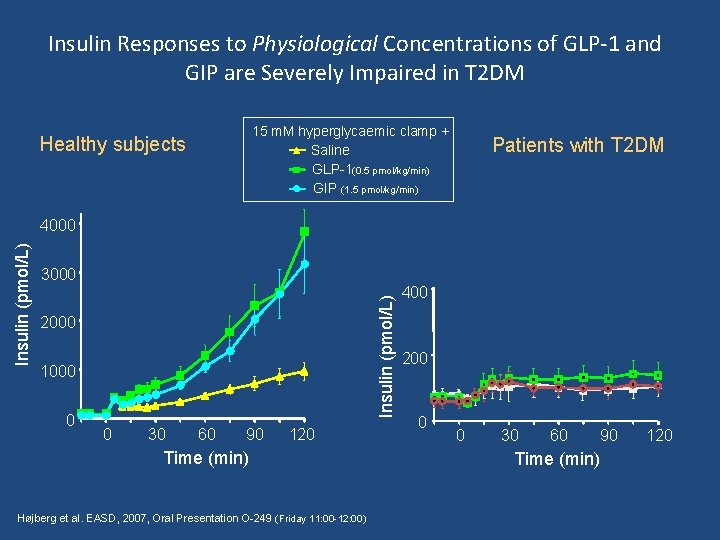
Insulin Responses to Physiological Concentrations of GLP-1 and GIP are Severely Impaired in T 2 DM 15 m. M hyperglycaemic clamp + Saline GLP-1(0. 5 pmol/kg/min) GIP (1. 5 pmol/kg/min) Healthy subjects Patients with T 2 DM 3000 Insulin (pmol/L) 4000 2000 1000 0 0 30 60 90 120 Time (min) Højberg et al. EASD, 2007, Oral Presentation O-249 (Friday 11: 00 -12: 00) 400 200 0 0 30 60 Time (min) 90 120
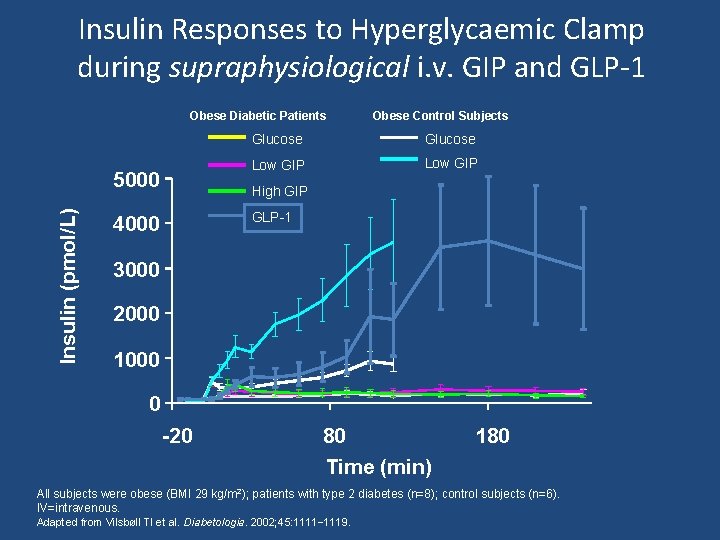
Insulin Responses to Hyperglycaemic Clamp during supraphysiological i. v. GIP and GLP-1 Obese Diabetic Patients Insulin (pmol/L) 5000 Obese Control Subjects Glucose Low GIP High GIP GLP-1 4000 3000 2000 1000 0 -20 80 Time (min) 180 All subjects were obese (BMI 29 kg/m 2); patients with type 2 diabetes (n=8); control subjects (n=6). IV=intravenous. Adapted from Vilsbøll Tl et al. Diabetologia. 2002; 45: 1111– 1119.

Why is Incretin function defective in type 2 diabetes? • Secretion of GLP-1 impaired • Beta-cell sensitivity to GLP-1 decreased, but supraphysiological amounts can normalise glucoseinduced insulin secretion • Secretion of GIP slightly impaired • Effect of GIP abolished or grossly impaired Toft Nielsen et al 1998 -2004 Vilsboll et al 2000 -2007 Højbjerg et al 2007
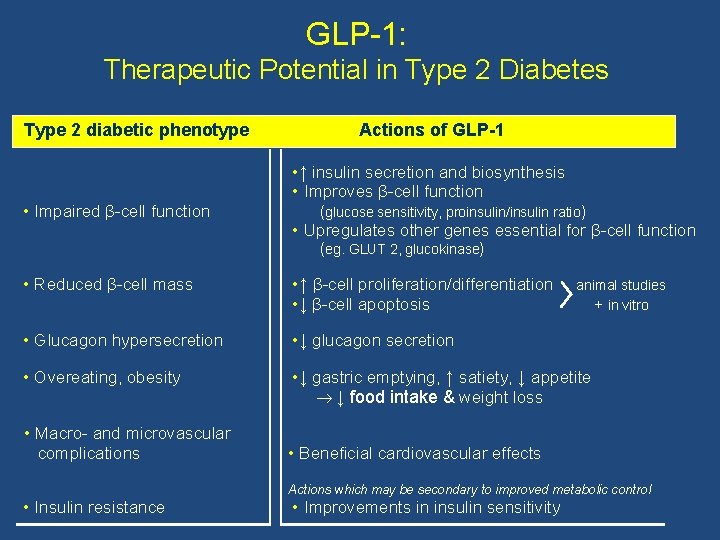
GLP-1: Therapeutic Potential in Type 2 Diabetes Type 2 diabetic phenotype • Impaired β-cell function Actions of GLP-1 • ↑ insulin secretion and biosynthesis • Improves β-cell function (glucose sensitivity, proinsulin/insulin ratio) • Upregulates other genes essential for β-cell function (eg. GLUT 2, glucokinase) • Reduced β-cell mass • ↑ β-cell proliferation/differentiation • ↓ β-cell apoptosis • Glucagon hypersecretion • ↓ glucagon secretion • Overeating, obesity • ↓ gastric emptying, ↑ satiety, ↓ appetite ↓ food intake & weight loss • Macro- and microvascular complications • Insulin resistance animal studies + in vitro • Beneficial cardiovascular effects Actions which may be secondary to improved metabolic control • Improvements in insulin sensitivity
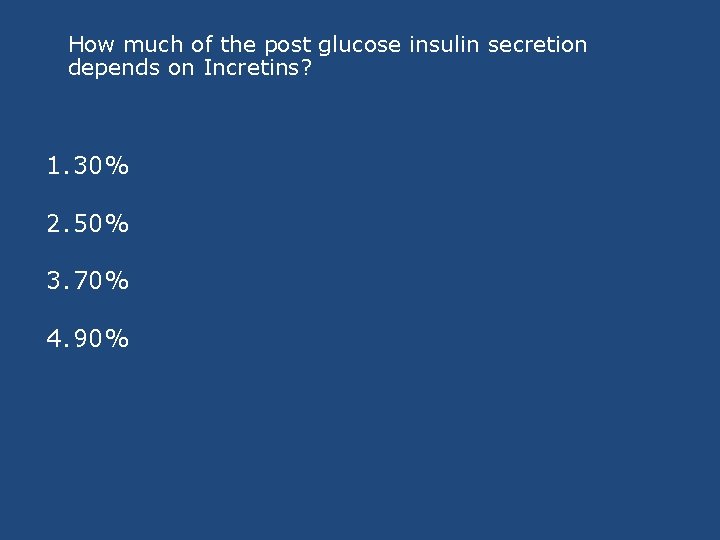
How much of the post glucose insulin secretion depends on Incretins? 1. 30% 2. 50% 3. 70% 4. 90%
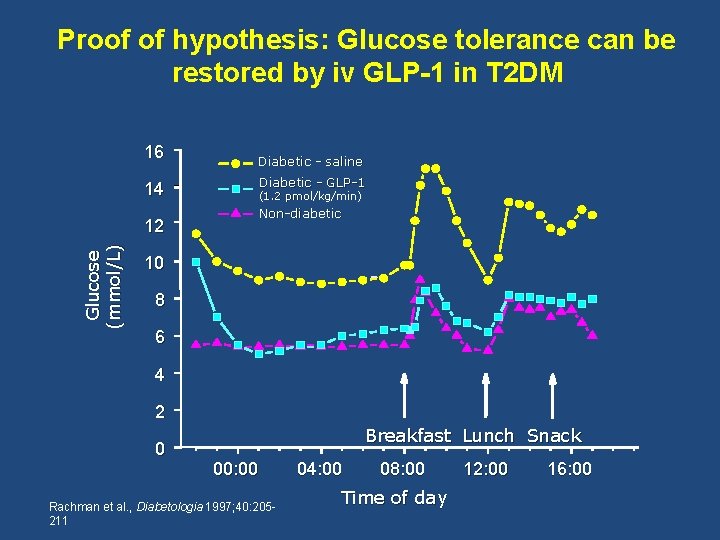
Proof of hypothesis: Glucose tolerance can be restored by iv GLP-1 in T 2 DM 16 14 Glucose (mmol/L) 12 Diabetic - saline Diabetic - GLP-1 (1. 2 pmol/kg/min) Non-diabetic 10 8 6 4 2 0 Breakfast Lunch Snack 00: 00 Rachman et al. , Diabetologia 1997; 40: 205211 04: 00 08: 00 Time of day 12: 00 16: 00
Effects of six weeks’ continuous subcutaneous infusion of GLP-1 in patients with type 2 diabetes Zander et al, Lancet 2002

Main findings: Zander et al. Lancet 2002 • Continuous subcutanous infusion of GLP-1 for 6 weeks in type 2 diabetes patients: – Reduced fasting and mean plasma glucose by 4. 3 and 5. 5 mmol/l, respectively – Reduced Hb. A 1 c by 1. 3% and normalised fructosamine – Resulted in a weight loss of 2 kg, presumably because of significantly reduced appetite – Improved insulin sensitivity and enhanced beta-cell secretion – Had no significant side effects – Suboptimal dose; suboptimal administration

How can we exploit therapeutic potential of GLP-1? Deacon CF, Nauck MA, Toft-Nielsen M-B, Pridal L, Willms B and Holst JJ: Both subcutaneously and intravenously administered GLP-1 are rapidly degraded from the NH 2 -terminus in type II diabetic patients and healthy subjects. Diabetes 1995; 44: 1126 -31. P. 1130: ”Inhibition of dipeptidylpeptidase IV may prove a useful adjunct in the management of type II diabetes, as has been the case for the ACE-inhibitors to treat hypertension”

How can we exploit therapeutic potential of GLP-1? GLP-1 receptor activators • Metabolically stable activators of the GLP-1 receptor (exendin derivatives: Byetta, Lixisenatide) • Slow release formulations of exendin or GLP-1 analogues (Bydureon and Taspoglutide) • Covalent (albiglutide, dulaglutide) or non-covalent (liraglutide) association of GLP-1 with large proteins (albumin – fc fragments) -> long half-lives • Inhibitors of DPP-4 (small orally active molecules) • Small molecule activators of GLP-1 R

GLP-1 receptor activators • Exendin 4, from saliva of the Gila Monster, 53% homologous with GLP-1 and full agonist on the GLP-1 receptor • Insensitive to DPP-IV • Cleared slowly by kidneys Gila Monster

Which one of the following statements is incorrect regarding Incretin function in type 2 diabetes? 1. GLP-1 secretion is impaired 2. Beta-cell sensitivity to physiological levels of GLP-1 is normal 3. GIP secretion is impaired 4. GIP effect is abolished or severely impaired
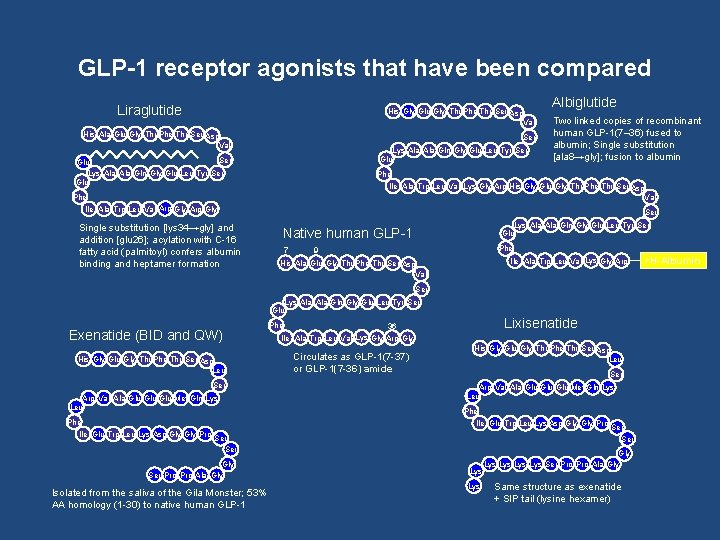
GLP-1 receptor agonists that have been compared Liraglutide His Gly Glu Gly Thr Phe Thr Ser Asp Val His Ala Glu Gly Thr Phe Thr Ser Asp Val Ser Lys Ala Gln Gly Glu Leu Tyr Ser Glu Albiglutide Two linked copies of recombinant human GLP-1(7– 36) fused to albumin; Single substitution [ala 8→gly]; fusion to albumin Phe Ile Ala Trp Leu Val Lys Gly Arg His Gly Glu Gly Thr Phe Thr Ser Asp Val Phe Ile Ala Trp Leu Val Arg Gly Single substitution [lys 34→gly] and addition [glu 26]; acylation with C-16 fatty acid (palmitoyl) confers albumin binding and heptamer formation Ser Lys Ala Gln Gly Glu Leu Tyr Ser Glu Native human GLP-1 7 Phe 9 Ile Ala Trp Leu Val Lys Gly Arg His Ala Glu Gly Thr Phe Thr Ser Asp Val Ser Lys Ala Gln Gly Glu Leu Tyr Ser Glu Exenatide (BID and QW) His Gly Glu Gly Thr Phe Thr Ser Asp Leu Ser Arg Val Ala Glu Glu Met Gln Lys Leu Phe Lixisenatide Phe 36 Ile Ala Trp Leu Val Lys Gly Arg Gly Circulates as GLP-1(7 -37) or GLP-1(7 -36) amide His Gly Glu Gly Thr Phe Thr Ser Asp Leu Ser Arg Val Ala Glu Glu Met Gln Lys Leu Phe Ile Glu Trp Leu Lys Asp Gly Gly Pro Ser Gly Ser Pro Ala Gly Isolated from the saliva of the Gila Monster; 53% AA homology (1 -30) to native human GLP-1 Lys Ser Gly Lys Lys Ser Pro Ala Gly Same structure as exenatide + SIP tail (lysine hexamer) r. H-Albumin
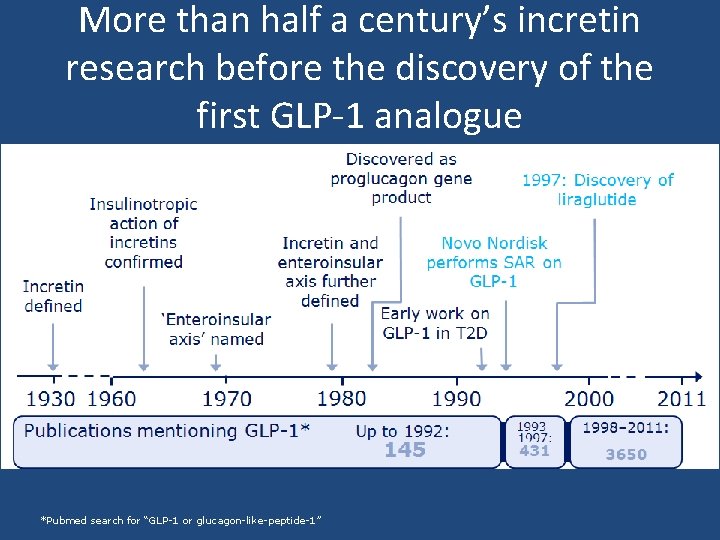
More than half a century’s incretin research before the discovery of the first GLP-1 analogue *Pubmed search for “GLP-1 or glucagon-like-peptide-1”
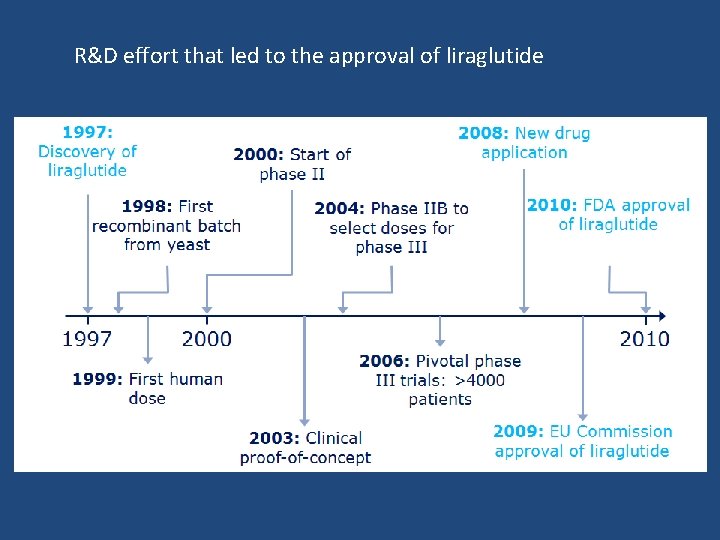
R&D effort that led to the approval of liraglutide
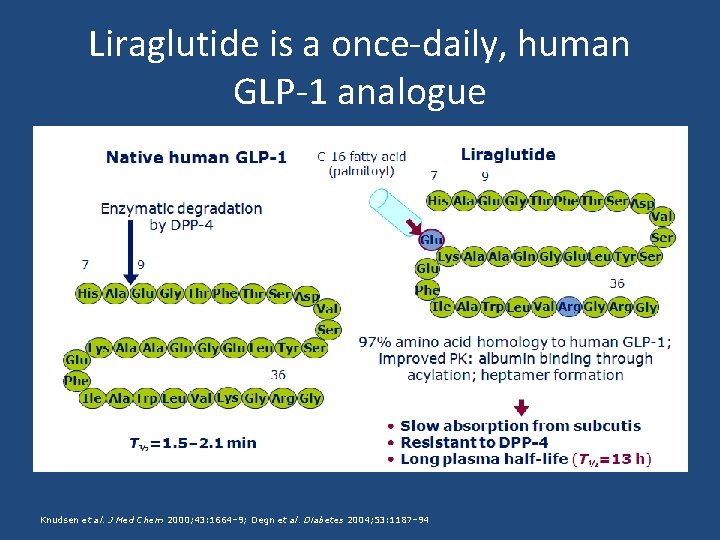
Liraglutide is a once-daily, human GLP-1 analogue Knudsen et al. J Med Chem 2000; 43: 1664– 9; Degn et al. Diabetes 2004; 53: 1187– 94
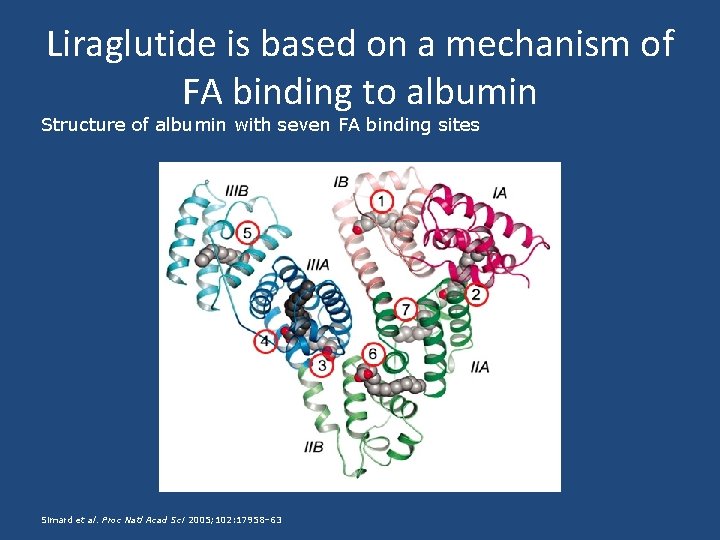
Liraglutide is based on a mechanism of FA binding to albumin Structure of albumin with seven FA binding sites Simard et al. Proc Natl Acad Sci 2005; 102: 17958– 63
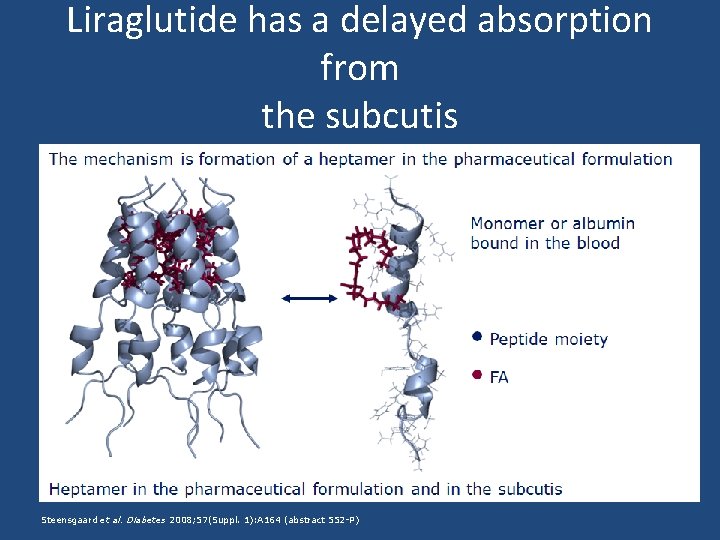
Liraglutide has a delayed absorption from the subcutis Steensgaard et al. Diabetes 2008; 57(Suppl. 1): A 164 (abstract 552 -P)
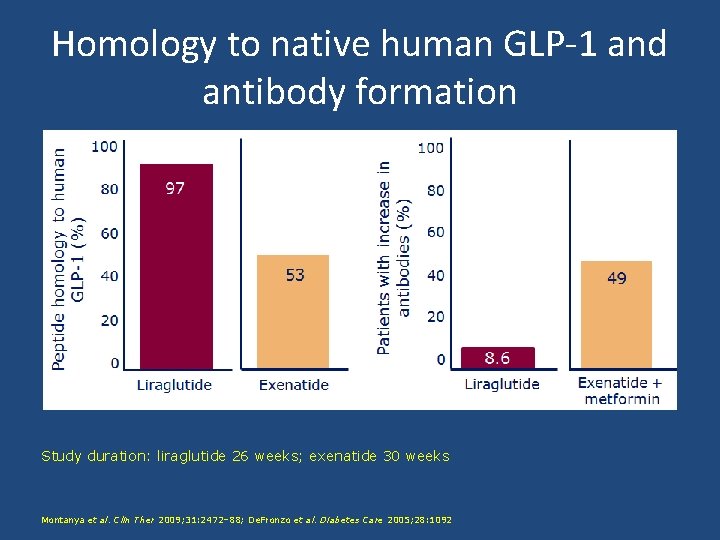
Homology to native human GLP-1 and antibody formation Study duration: liraglutide 26 weeks; exenatide 30 weeks Montanya et al. Clin Ther 2009; 31: 2472– 88; De. Fronzo et al. Diabetes Care 2005; 28: 1092
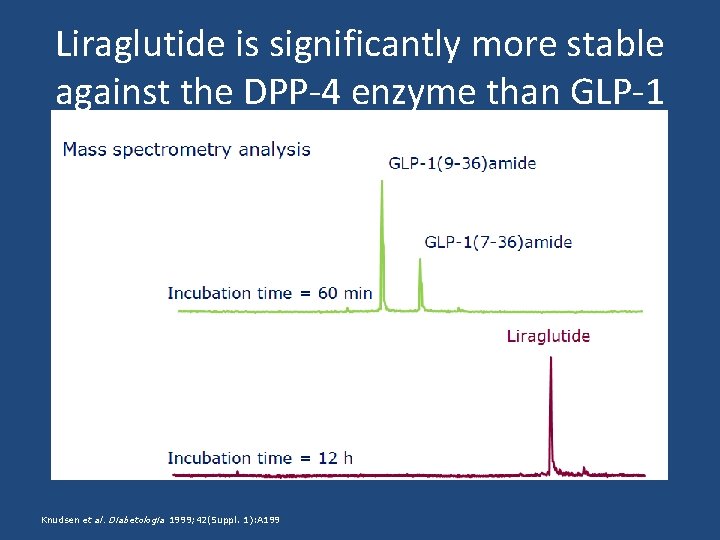
Liraglutide is significantly more stable against the DPP-4 enzyme than GLP-1 Knudsen et al. Diabetologia 1999; 42(Suppl. 1): A 199

What is plasma half life of liraglutide? 1. 13 h 2. 10 h 3. 20 h 4. 8 h
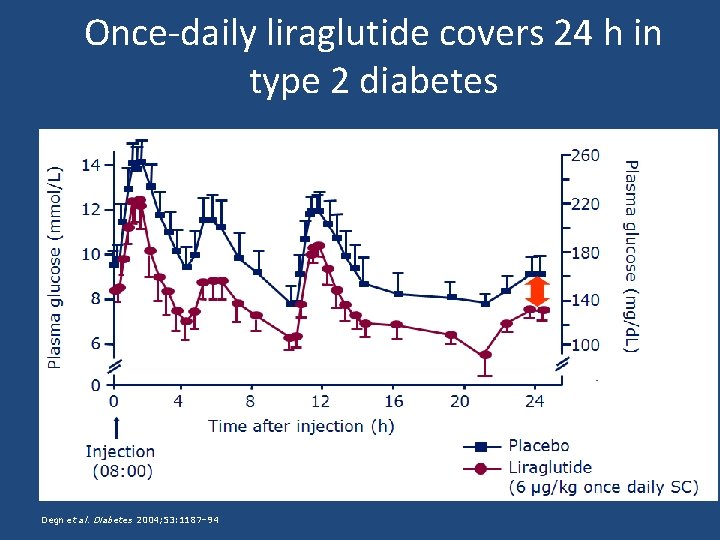
Once-daily liraglutide covers 24 h in type 2 diabetes Degn et al. Diabetes 2004; 53: 1187– 94
What percentage of patients develope anti liraglutide antibody? ? 1. 30% 2. 49% 3. 8. 6% 4. 15%
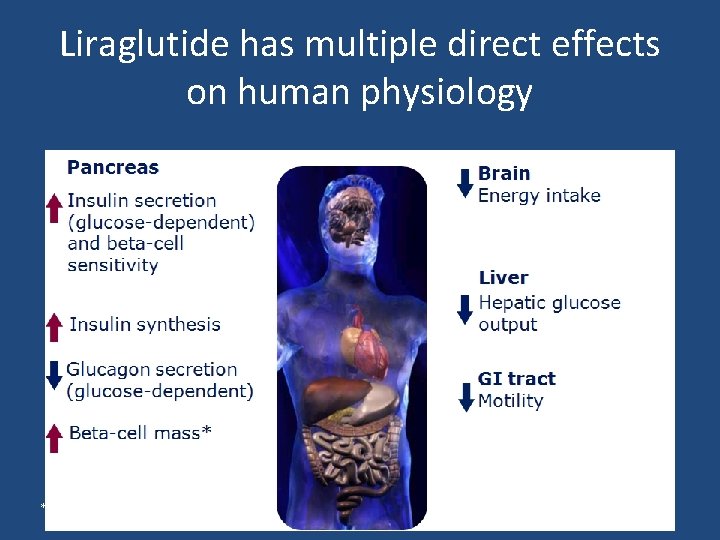
Liraglutide has multiple direct effects on human physiology *In animal studies
Liraglutide provides multiple clinical benefits • • Liraglutide is the first once-daily, human GLP-1 analogue to provide glycaemic control throughout the continuum of care in type 2 diabetes Liraglutide provides: – substantial and sustained reduction in blood glucose levels – clinically significant weight loss – reduction in SBP – improvement in beta-cell function Liraglutide SPC

Overview of LEAD Trials F. Hosseinpanah, M. D. Obesity Research Center Research Institute for Endocrine sciences Shahid Beheshti University of Medical Sciences May 22, 2014 Tehran

Liraglutide Effect and Action in Diabetes • A series of six randomized controlled phase 3 trials, which was conducted at more than 600 sites in 40 countries involving more than 4000 patients, of whom approximately 2700 received liraglutide

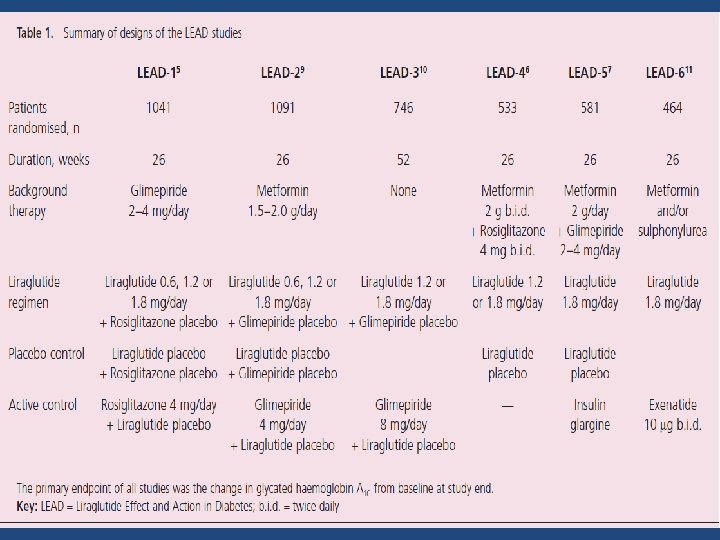
Design of the LEAD Trials • Primary objective : To assess the effect of liraglutide on glycemic control as measured by change in Hb. A 1 c • All trials were randomized, parallel group, multicenter trials • Three different dose levels of liraglutide (0. 6 mg, 1. 2 mg and 1. 8 mg/day) were evaluated • The duration of all LEAD trials was 26 weeks, except LEAD 3, which was 52 weeks
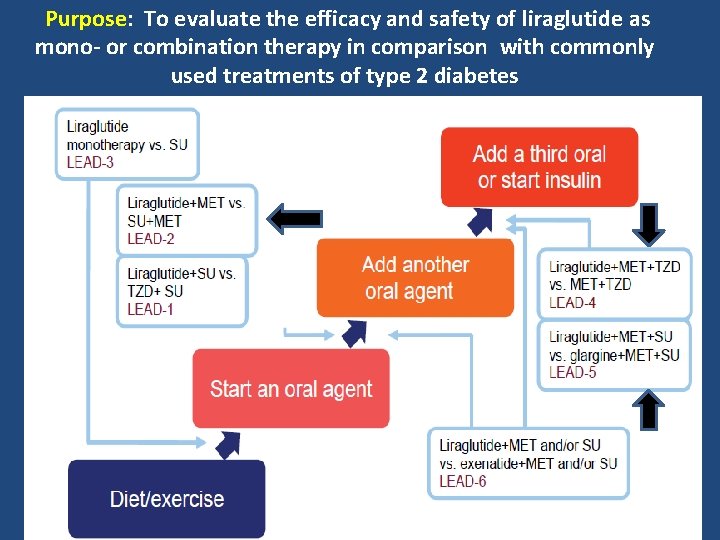
Purpose: To evaluate the efficacy and safety of liraglutide as mono- or combination therapy in comparison with commonly used treatments of type 2 diabetes
• 26 -week, double-blind, double-dummy • 1, 091 subjects were randomly assigned (2: 2: 2: 1: 2) to once-daily liraglutide (either 0. 6, 1. 2, or 1. 8 mg/day injected subcutaneously), to placebo, or to glimepiride (4 mg once daily) • All treatments were in combination therapy with metformin (1 g twice daily) Diabetes Care 32: 84– 90, 2009
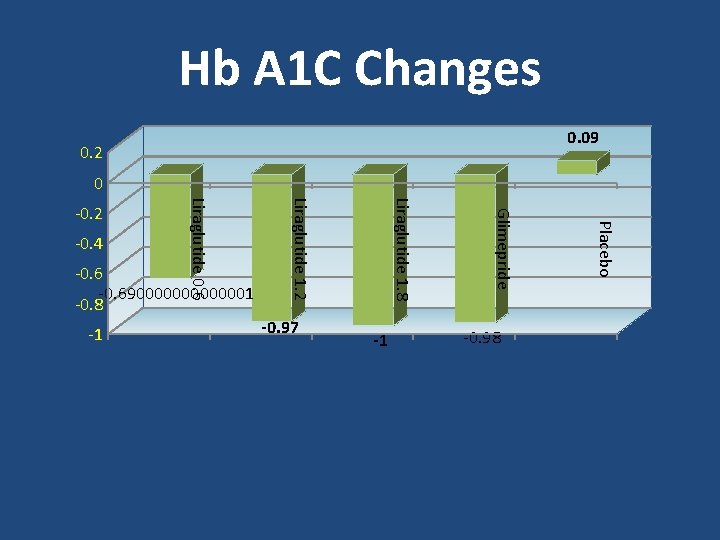
Hb A 1 C Changes 0. 09 0. 2 0 -1 -0. 98 Placebo -0. 97 Glimepride -1 Liraglutide 1. 8 -0. 690000001 -0. 8 Liraglutide 1. 2 -0. 4 Liraglutide 0. 6 -0. 2
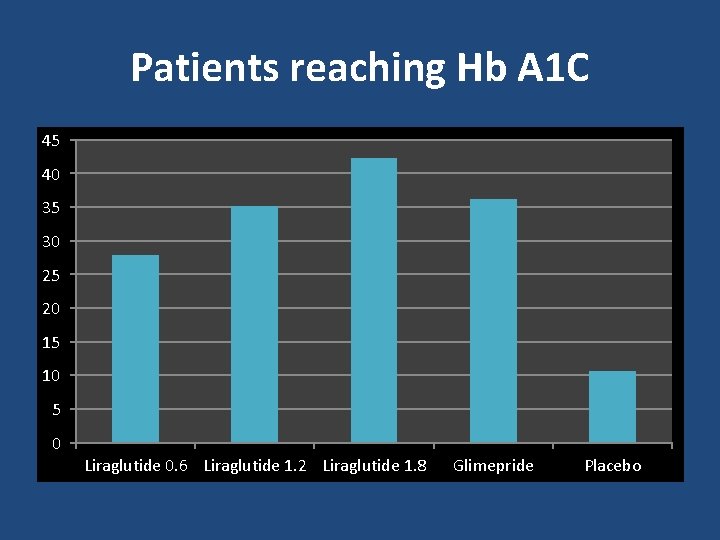
Patients reaching Hb A 1 C 45 40 35 30 25 20 15 10 5 0 Liraglutide 0. 6 Liraglutide 1. 2 Liraglutide 1. 8 Glimepride Placebo
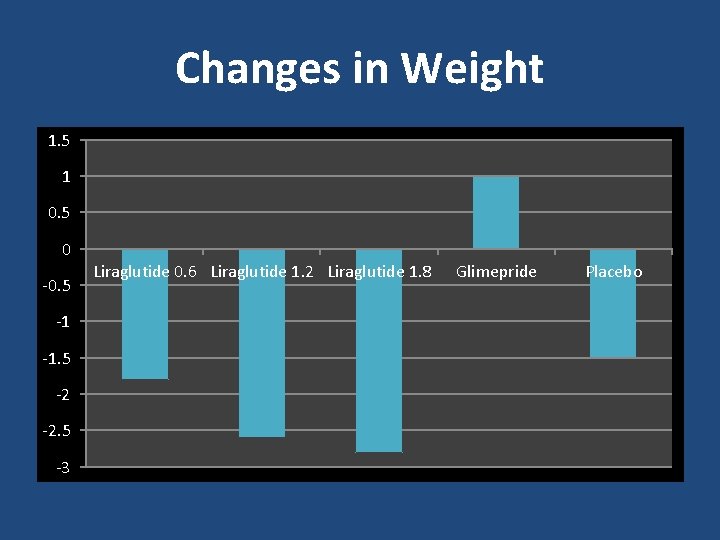
Changes in Weight 1. 5 1 0. 5 0 -0. 5 -1 -1. 5 -2 -2. 5 -3 Liraglutide 0. 6 Liraglutide 1. 2 Liraglutide 1. 8 Glimepride Placebo

• 26 -week, double-blind, placebo-controlled randomized trial • 533 subjects randomized(1: 1: 1) to once-daily liraglutide (1. 2 or 1. 8 mg) or liraglutide placebo in combination with metformin (1 g twice daily) and rosiglitazone (4 mg twice daily) Diabetes Care 32: 1224– 1230, 2009
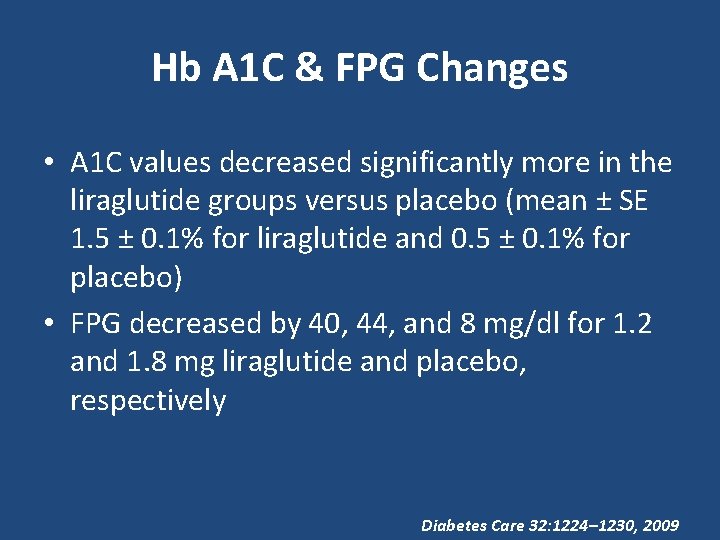
Hb A 1 C & FPG Changes • A 1 C values decreased significantly more in the liraglutide groups versus placebo (mean ± SE 1. 5 ± 0. 1% for liraglutide and 0. 5 ± 0. 1% for placebo) • FPG decreased by 40, 44, and 8 mg/dl for 1. 2 and 1. 8 mg liraglutide and placebo, respectively Diabetes Care 32: 1224– 1230, 2009
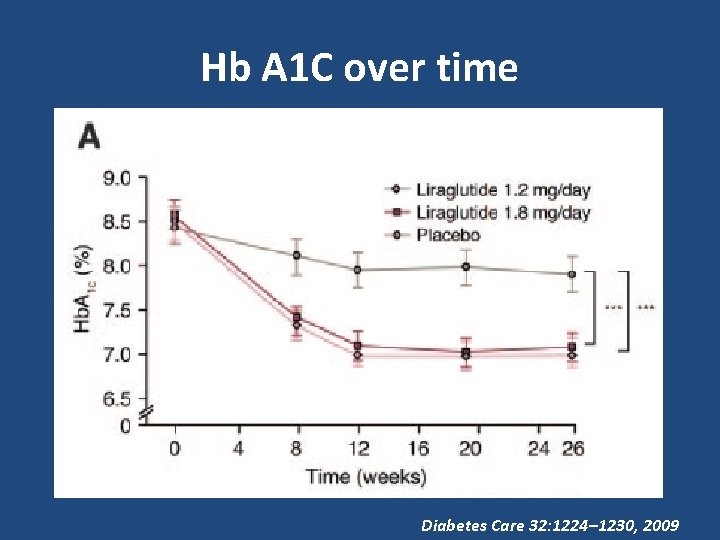
Hb A 1 C over time Diabetes Care 32: 1224– 1230, 2009
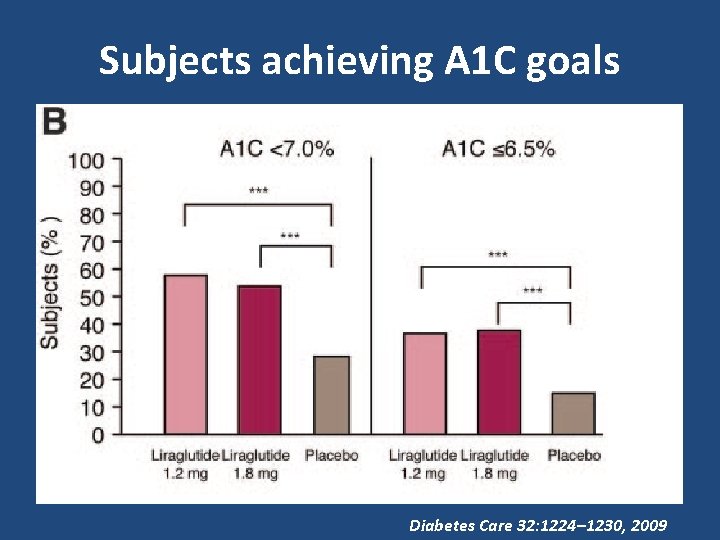
Subjects achieving A 1 C goals Diabetes Care 32: 1224– 1230, 2009
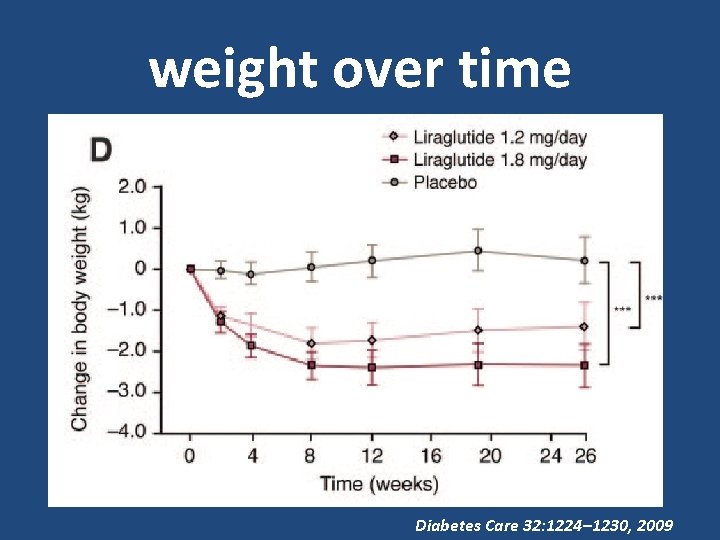
weight over time Diabetes Care 32: 1224– 1230, 2009
• 581 patients with type 2 diabetes mellitus on prior OHA for at least 3 months (Hb. A 1 c 7– 10%) • Patients were randomized (2: 1: 2) to liraglutide 1. 8 mg once daily (n=232), placebo (n=115) and open-label insulin glargine (n=234), all in combination with metformin (1 g twice daily) and glimepiride (4 mg once daily) Diabetologia (2009) 52: 2046– 2055
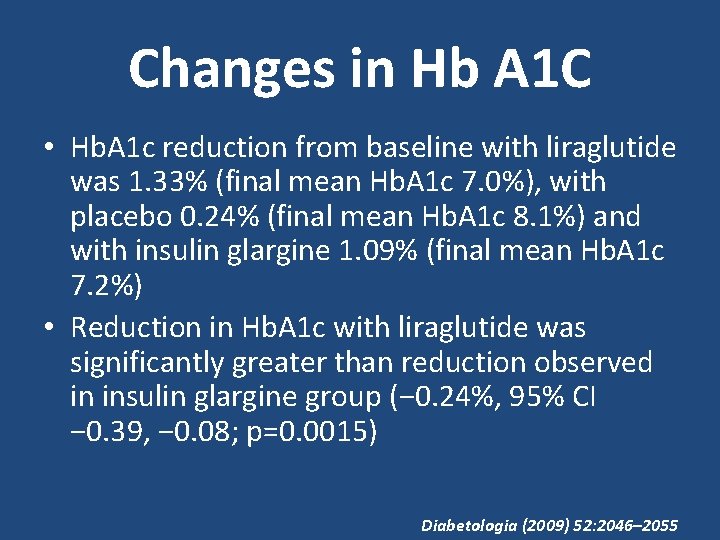
Changes in Hb A 1 C • Hb. A 1 c reduction from baseline with liraglutide was 1. 33% (final mean Hb. A 1 c 7. 0%), with placebo 0. 24% (final mean Hb. A 1 c 8. 1%) and with insulin glargine 1. 09% (final mean Hb. A 1 c 7. 2%) • Reduction in Hb. A 1 c with liraglutide was significantly greater than reduction observed in insulin glargine group (− 0. 24%, 95% CI − 0. 39, − 0. 08; p=0. 0015) Diabetologia (2009) 52: 2046– 2055
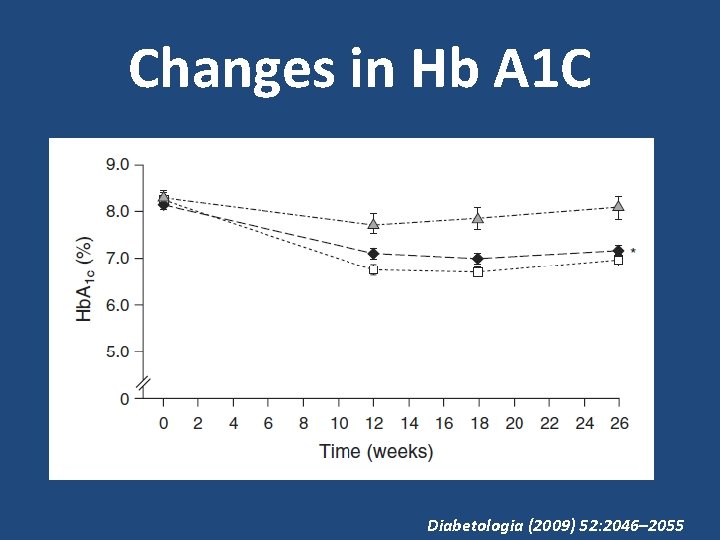
Changes in Hb A 1 C Diabetologia (2009) 52: 2046– 2055
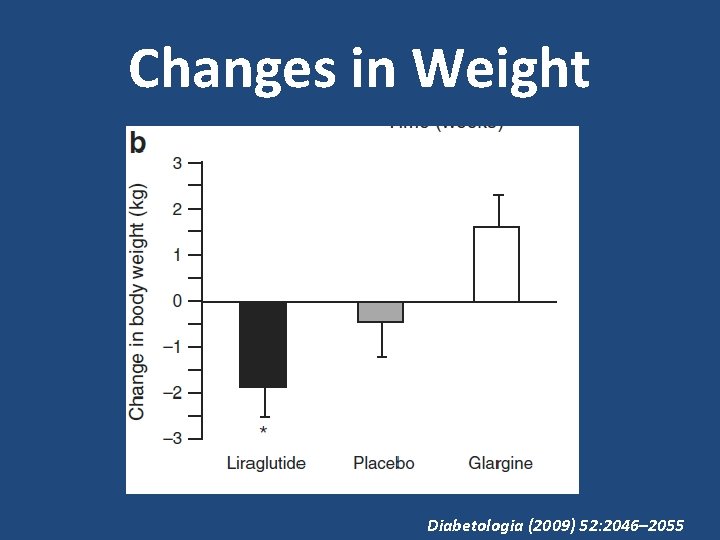
Changes in Weight Diabetologia (2009) 52: 2046– 2055
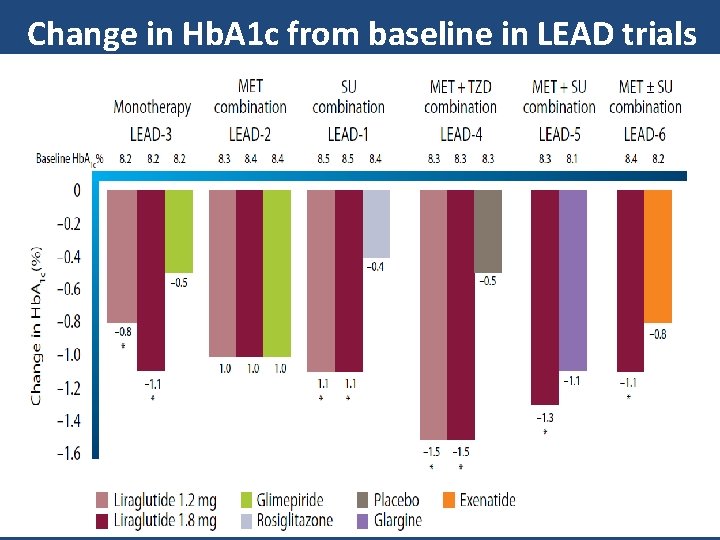
Change in Hb. A 1 c from baseline in LEAD trials
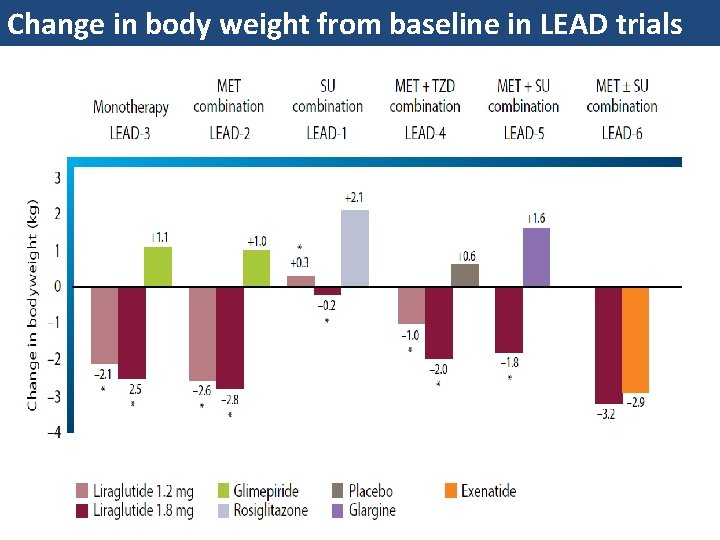
Change in body weight from baseline in LEAD trials
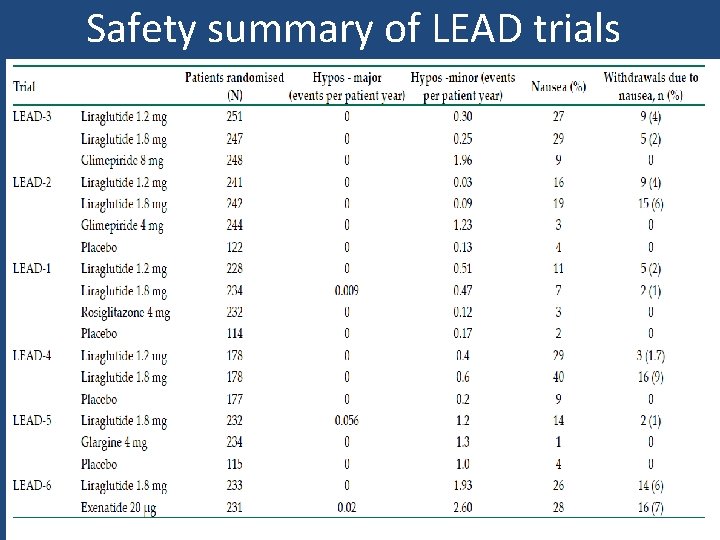
Safety summary of LEAD trials

LEADER • LEADER® is an international study that investigates a once-daily diabetes treatment developed by Novo Nordisk, and the effect it has on heart disease in people with type 2 diabetes. • The study involves around 9, 000 people with type 2 diabetes from more than 30 countries worldwide. It started in September 2010 and will run for approximately five years (01/2016)
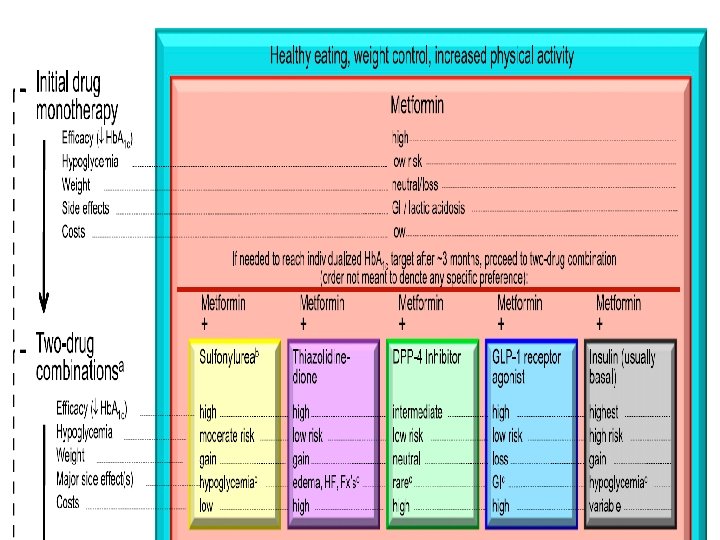
Liraglutide is effective across the continuum of care in type 2 diabetes • Liraglutide is effective across the continuum of care – – as monotherapy after one oral antidiabetic drug (OAD) after two OADs prior to basal insulin therapy Marre et al. Diabetic Medicine 2009; 26: 268– 78 (LEAD-1); Nauck et al. Diabetes Care 2009; 32: 84– 90 (LEAD-2); Garber et al. Lancet 2009; 373: 473– 81 (LEAD-3); Zinman et al. Diabetes Care 2009; 32: 1224– 30 (LEAD-4); Russell-Jones et al. Diabetologia 2009; 52: 2046– 55 (LEAD-5); Buse et al. Lancet 2009; 374: 39– 47 (LEAD-6)
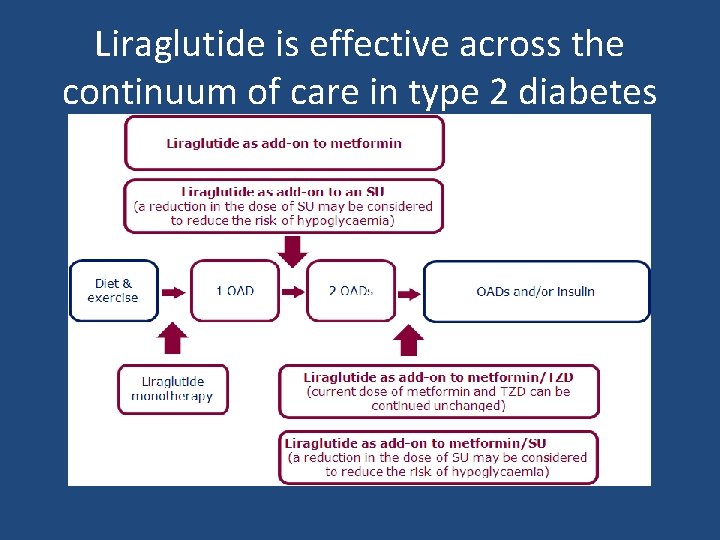
Liraglutide is effective across the continuum of care in type 2 diabetes

Liraglutide administration • • • 24 -h glucose control once-daily dosing can be given at any time of the day dosing independent of meals no need for additional self-monitored plasma glucose

Based on the LEAD studies, liraglutide offers simple dose initiation and titration
Conclusions • Incretin hormone secretion and actions are impaired in type 2 diabetes. • β-cell responsiveness to GLP-1 is reduced, but larger amounts of GLP 1 can still restore β-cell sensitivity to glucose and improve glucoseinduced insulin and glucagon secretion. • This can be obtained both with - incretin mimetics (GLP-1 receptor activators) or - incretin enhancers (DPP-4 inhibitors) • Incretin-based therapies of type 2 diabetes may be expected to – – Cause sustained improvements in glycaemia and Hb. A 1 c levels Improve α-cell and β-cell function Improve insulin sensitivity Improve metabolism

Conclusions • Liraglutide is effective across the continuum of care in type 2 diabetes • Liraglutide administration – – – 24 -h glucose control once-daily dosing can be given at any time of the day dosing independent of meals no need for additional self-monitored plasma glucose • Liraglutide offers a simple initiation, titration and maintenance regimen

Conclusion • Liraglutide, as mono or combination therapy with a range of anti diabetic drugs, can lead to significant improvements in Hb. A 1 c, FPG and PPG, while reducing body weight and SBP significantly • Liraglutide is generally safe and well tolerated, and associated with a low rate of hypoglycemia with mild, transient nausea as the most commonly reported adverse event
- Slides: 62