Increasing transrectal ultrasound guided prostate biopsy associated infection
Increasing transrectal ultrasound guided prostate biopsy associated infection; is a change in antimicrobial prophylaxis the solution? Authors: Ni Bhuachalla C 1, Mc. Namara E 2, Carroll A 2, Lynch T 1, Boyle B 1 1. St James’s Hospital, Dublin 8, Ireland. 2. Public Health Laboratory HSE, Dublin 10, Ireland.

TRUS biopsy associated infection • Surveillance/ diagnostic protocol • Post procedure infection rates Ø 1 - 3% UTI Ø 0. 5 - 1% BSI • Strategies to reduce rate of infection ØAntimicrobial prophylaxis ØPre procedure screening ØDecontamination protocol ØOperator training
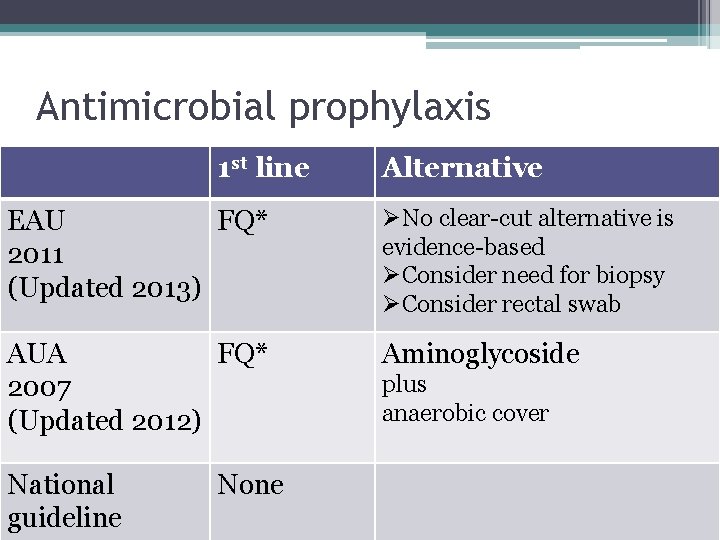
Antimicrobial prophylaxis 1 st line Alternative EAU FQ* 2011 (Updated 2013) ØNo clear-cut alternative is evidence-based ØConsider need for biopsy ØConsider rectal swab AUA FQ* 2007 (Updated 2012) Aminoglycoside National guideline None plus anaerobic cover
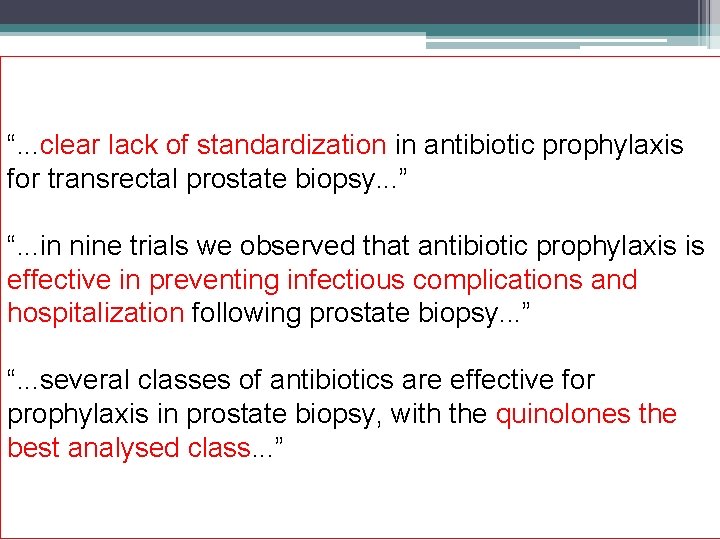
“. . . clear lack of standardization in antibiotic prophylaxis for transrectal prostate biopsy. . . ” “. . . in nine trials we observed that antibiotic prophylaxis is effective in preventing infectious complications and hospitalization following prostate biopsy. . . ” “. . . several classes of antibiotics are effective for prophylaxis in prostate biopsy, with the quinolones the best analysed class. . . ”

Antimicrobial resistance • • prevalence fluoroquinolone (FQ) resistance prevalence gentamicin resistance Previous exposure to antimicrobials Previous health care contact
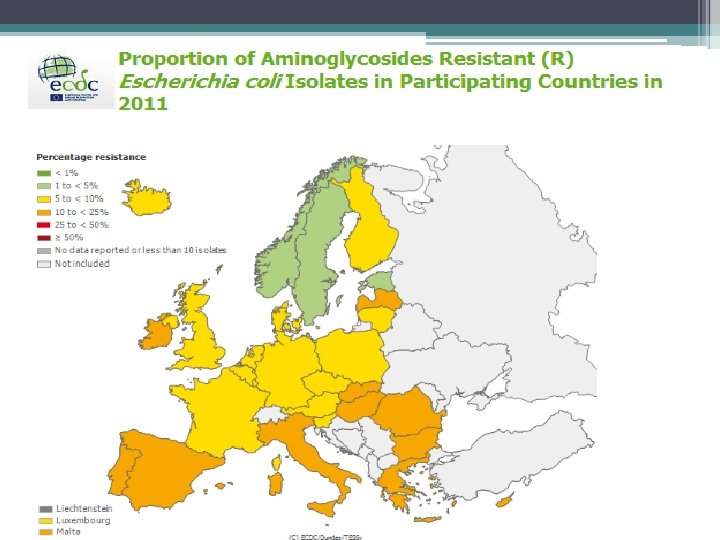
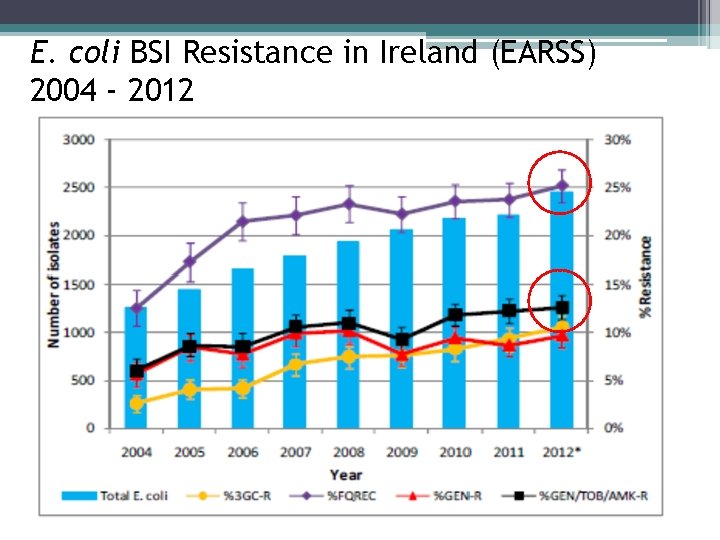
E. coli BSI Resistance in Ireland (EARSS) 2004 - 2012

E. coli BSI resistance St James’s Hospital 2009 - 2012 % Resistance


Aims • To identify all patients with post TRUS biopsy infection from Jan 2010 - Nov 2012 • Review isolates and susceptibilities causing infection • Review antimicrobial prophylaxis administered • To assess efficacy of change in prophylaxis from Dec 2012 to date
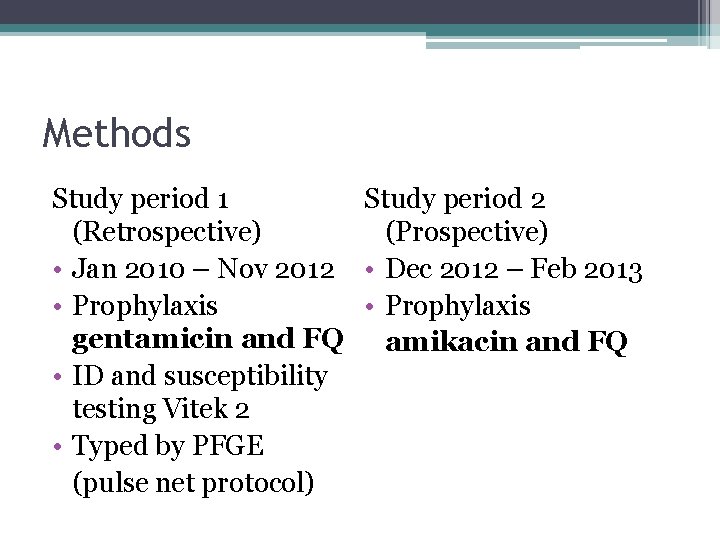
Methods Study period 1 Study period 2 (Retrospective) (Prospective) • Jan 2010 – Nov 2012 • Dec 2012 – Feb 2013 • Prophylaxis gentamicin and FQ amikacin and FQ • ID and susceptibility testing Vitek 2 • Typed by PFGE (pulse net protocol)

Study period 1 Jan 2010 – Nov 2012 Isolates No. TRUS biopsies conducted All E. coli 1398 Ciprofloxacin resistance Overall infection rate 73. 3% (11/15) 1% (15) Gentamicin resistance Type of post biopsy infection 40% (4/10) 0. 4 %BSI/ SSI (6/15) 0. 6% UTI (9/15) Amikacin Isolates resistance 0% All(5/5) E. coli Previous TRUS biopsy ESBL producer (9/15) 6. 7%60% (1/15)
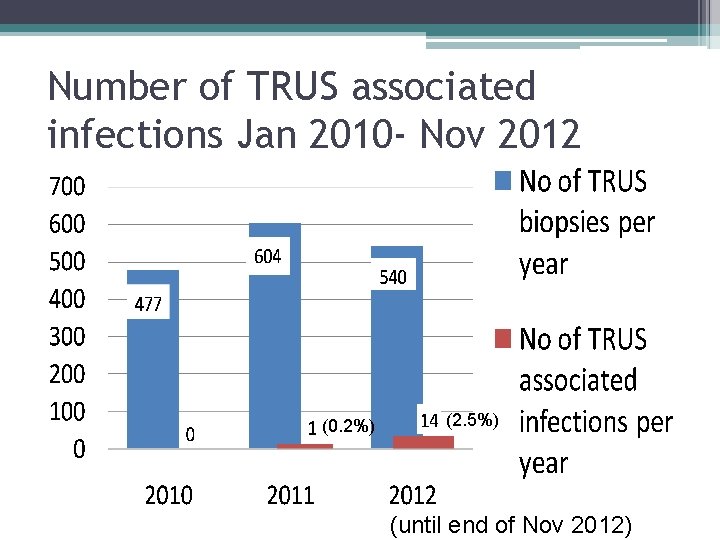
Number of TRUS associated infections Jan 2010 - Nov 2012 (0. 2%) (2. 5%) (until end of Nov 2012)

Study period 1 Jan 2010 – Nov 2012 PFGE • 6 available isolates - distinguishable
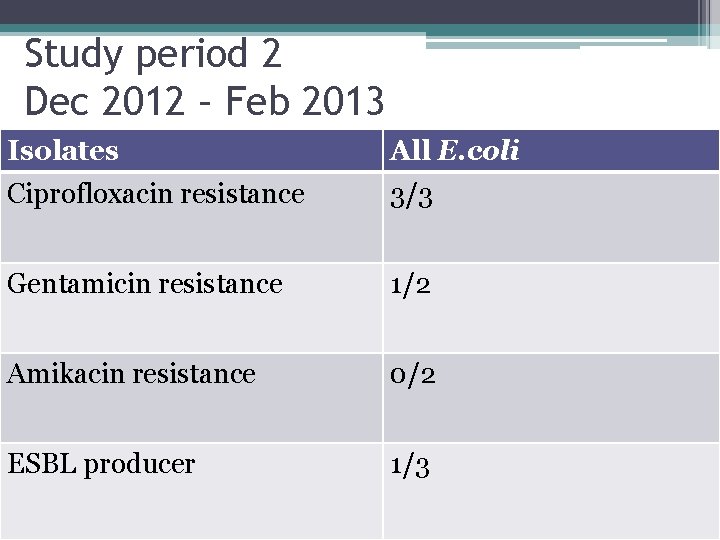
Study period 2 Dec 2012 – Feb 2013 Isolates No. TRUS biopsies conducted Ciprofloxacin resistance Overall infection rate All 140 E. coli 3/3 2% (3) Gentamicin resistance 1/2 Type of post biopsy infection 1. 3% BSI (2) 0. 7% UTI (1) Amikacin resistance 0/2 Isolates All E. coli Previous TRUS biopsy ESBL producer 1/32/3
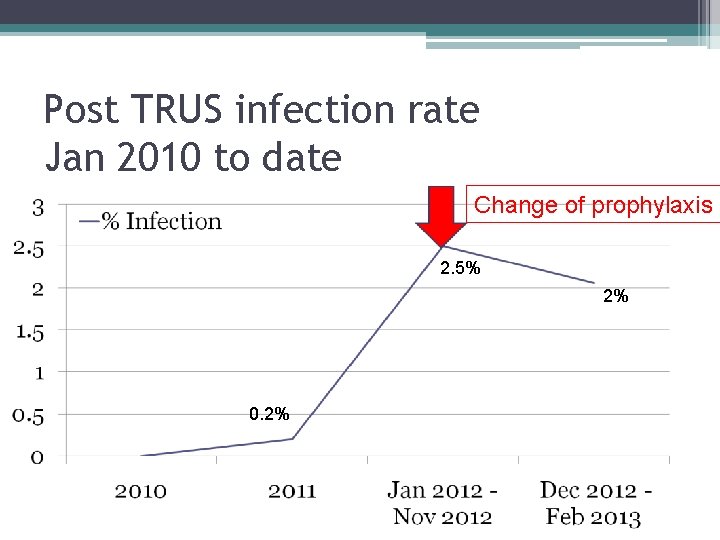
Post TRUS infection rate Jan 2010 to date Change of prophylaxis 2. 5% 2% 0. 2%



Local interim Recommendations Ø Amikacin and FQ? Ø Avoid fluoroquinolone use in empiric treatment of post biopsy infection Ø Targeted treatment of post biopsy infection when susceptibilities available Ø Targeted prophylaxis?
Conclusions Ø Ø Increase in TRUS biopsy associated infection Increasing antimicrobial resistance Prospective surveillance Potentially multifactorial process
- Slides: 20