Increasing the concentration of a reactant substance will


� Increasing the concentration of a reactant substance will increase the rate of reaction. This is because more collisions will occur, and therefore more successful collisions.
� Increasing the concentration of a reactant substance will increase the rate of reaction. This is because more collisions will occur, and therefore more successful collisions. � Eg – we used HCl of 2 M the other day and it would cause a faster reaction than 1 M because it has a higher concentration.

� If surface area is increased – more particles are exposed for collisions to occur.

� If surface area is increased – more particles are exposed for collisions to occur. The rate of reaction will increase as there are more collisions, therefore more successful collisions.
� If surface area is increased – more particles are exposed for collisions to occur. The rate of reaction will increase as there are more collisions, therefore more successful collisions. � EG – Calcium carbonate powder reacted much faster in HCl than Calcium carbonate chips. This is because the powder has a larger surface area.
� When temperature increases, particles move faster and collide more often.
� When temperature increases, particles move faster and collide more often. � The particles also have more energy to collide with = more products.

�A catalyst is a chemical or substance that is added to a reaction to speed it up.

�A catalyst is a chemical or substance that is added to a reaction to speed it up. � The catalyst does not change the products formed and doesn’t get used up in the reaction (so can be used again).

�A catalyst is a chemical or substance that is added to a reaction to speed it up. � The catalyst does not change the products formed and doesn’t get used up in the reaction (so can be used again). � The reaction rate increases because the catalyst lowers the activation energy needed for the reaction to occur.

� Set up two test tubes in a holder � Pour same amounts of hydrogen peroxide into them (about 2 cm) � In one test tube, put a small amount of manganese dioxide � Light a splint and shake it out (so it’s glowing) � Place it over the test tube WITHOUT the manganese dioxide – what happens? � Now, repeat it, and place the glowing splint over the other test tube – what happens?

� Hydrogen peroxide decomposes slowly to form water and oxygen gas. � If a catalyst (manganese dioxide) is added, a rapid supply of oxygen gas is produced

� Hydrogen peroxide decomposes slowly to form water and oxygen gas. � If a catalyst (manganese dioxide) is added, a rapid supply of oxygen gas is produced � Hydrogen peroxide water + oxygen
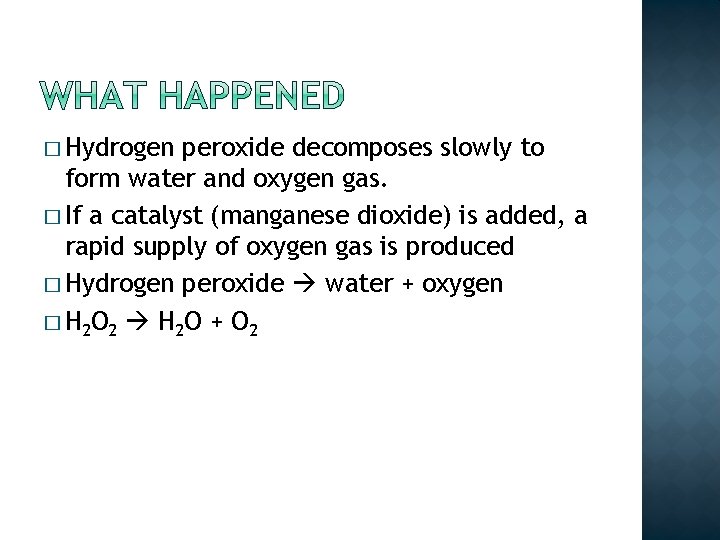
� Hydrogen peroxide decomposes slowly to form water and oxygen gas. � If a catalyst (manganese dioxide) is added, a rapid supply of oxygen gas is produced � Hydrogen peroxide water + oxygen � H 2 O 2 H 2 O + O 2
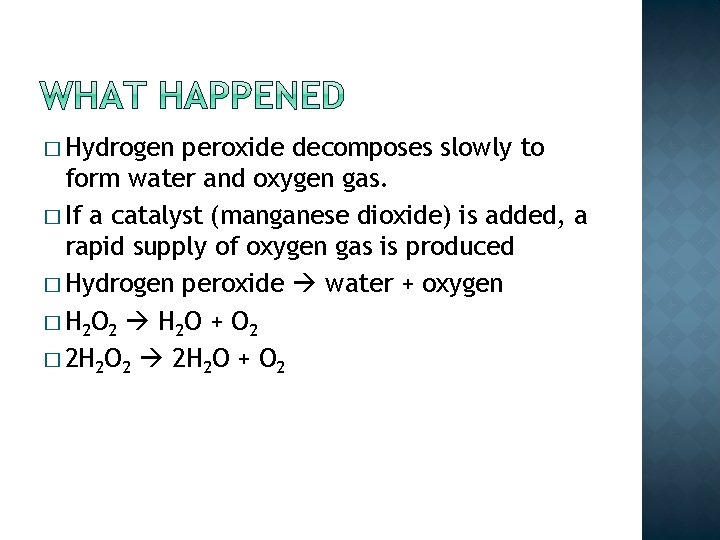
� Hydrogen peroxide decomposes slowly to form water and oxygen gas. � If a catalyst (manganese dioxide) is added, a rapid supply of oxygen gas is produced � Hydrogen peroxide water + oxygen � H 2 O 2 H 2 O + O 2 � 2 H 2 O 2 2 H 2 O + O 2
- Slides: 16