Incomplete Electron transfer in ionic compounds Intermediate Type












- Slides: 21

Incomplete Electron transfer in ionic compounds Intermediate Type of Bonding

Nature of pure ionic compounds Ways to get the value of lattice enthalpy Comparison of Theoretical and Experimental value of lattice enthalpy Polarization of ion

Nature of pure ionic compounds formed by complete transfer of electrons from metallic atoms to non-metallic atoms. electrons should be solely confined and not shared with its neighbouring ions In reality, there are some compounds that the transfer of electrons is incomplete.

Lattice Enthalpy of Ionic Compounds Lattice enthalpy: ³ Enthalpy change when one mole of ionic crystal is formed from its constituent ions at gaseous state under standard conditions How can we get the value of lattice enthalpy? ³ ³ By Energetics (Experimentally derived) By using the Simple Ionic Model (Theoretical)

By Energetics (Experimentally derived) We need to construct Born-Haber cycle and apply the Hess’s Law Let’s use Na. Cl as an example and calculate the lattice enthalpy

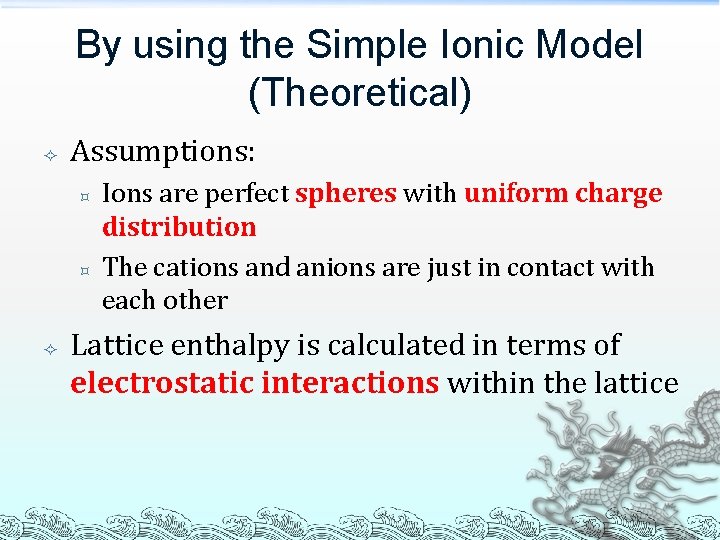
By using the Simple Ionic Model (Theoretical) Assumptions: ³ ³ Ions are perfect spheres with uniform charge distribution The cations and anions are just in contact with each other Lattice enthalpy is calculated in terms of electrostatic interactions within the lattice

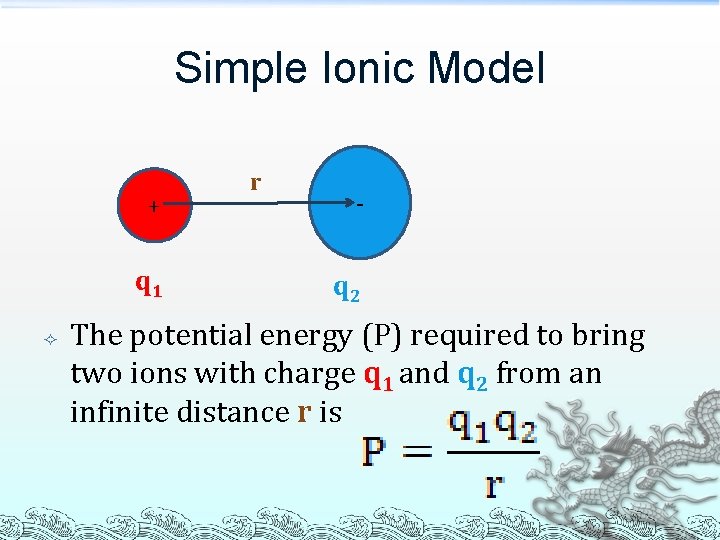
Simple Ionic Model + q 1 r - q 2 The potential energy (P) required to bring two ions with charge q 1 and q 2 from an infinite distance r is

Comparison of the Theoretical and Experimental Values of Lattice Enthalpy Reveals the nature of the bond in the compound

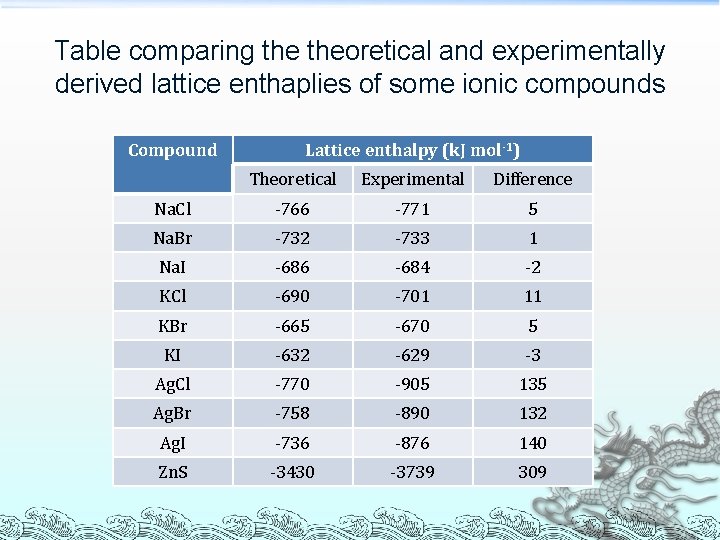
Table comparing theoretical and experimentally derived lattice enthaplies of some ionic compounds Compound Lattice enthalpy (k. J mol-1) Theoretical Experimental Difference Na. Cl -766 -771 5 Na. Br -732 -733 1 Na. I -686 -684 -2 KCl -690 -701 11 KBr -665 -670 5 KI -632 -629 -3 Ag. Cl -770 -905 135 Ag. Br -758 -890 132 Ag. I -736 -876 140 Zn. S -3430 -3739 309

Answer for Discussion For Na. Cl to KI, the differences between theoretical and experimentally derived lattice enthapies are small ³ The Simple Ionic Model gives a good representation of the actual lattice structure Ions are spherical ® Charge distribution is uniform ® ³ Bond type in these compounds is nearly purely ionic

Answer for Discussion For Ag. Cl, Ag. Br, Ag. I and Zn. S, there is a large difference between theoretical and experimentally derived lattice enthapies ³ Assumptions of the Simple Ionic Model are unsatisfactory Ions are not perfect spheres ® Charge distribution is not uniform ® ³ Bond type in these compounds is not purely ionic and has some degree of covalent character

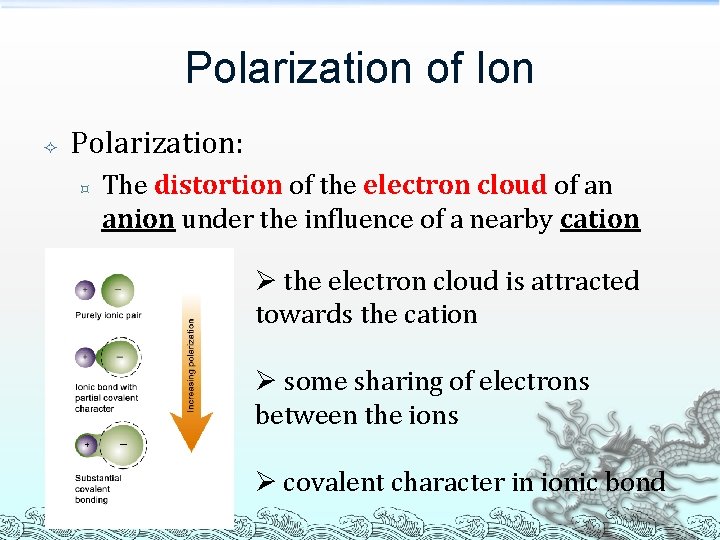
Polarization of Ion Polarization: ³ The distortion of the electron cloud of an anion under the influence of a nearby cation Ø the electron cloud is attracted towards the cation Ø some sharing of electrons between the ions Ø covalent character in ionic bond

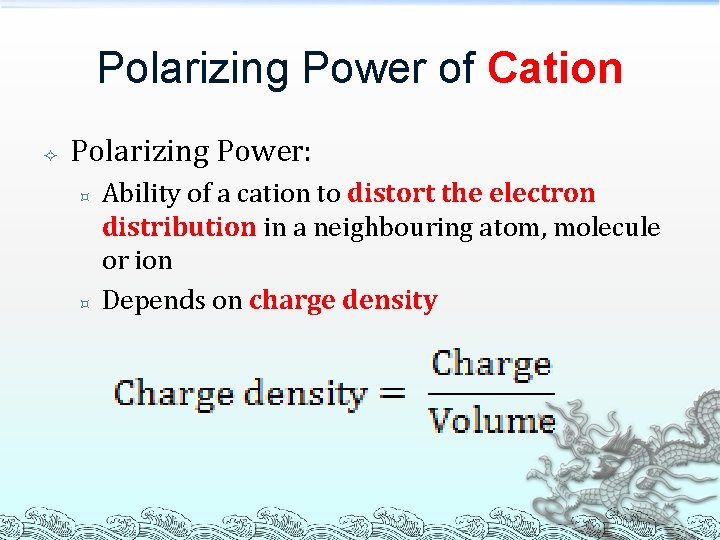
Polarizing Power of Cation Polarizing Power: ³ ³ Ability of a cation to distort the electron distribution in a neighbouring atom, molecule or ion Depends on charge density

The charge density of a cation is high if: ³ ³ Higher the charge; and/or Smaller the ionic radius The higher the charge density of a cation, the higher is its polarizing power

Think about: 1. Among Na+, Mg 2+ and Al 3+, which one has the highest polarizing power? Why? 2. Among Li+, Na+ and K+, which one has the highest polarizing power? Why?

Answer to Q 1 Al 3+ has the highest polarizing power ³ ³ It has the highest charge and smallest size among Na+, Mg 2+ and Al 3+ The charge density of Al 3+ is the greatest

Answer to Q 2 Li+ has the highest polarizing power ³ ³ ³ Among Li+, Na+ and K+, Li+ has the smallest size The charge density of Li+ is the highest Li+ has the highest polarizing power

Polarizability of Anion Polarizability: ³ ³ A measure of the ease of distortion of its electron cloud by neighbouring cations Higher polarizability of an anion if: Higher the charge; and/or ® The larger the ionic radius of anion ®

Think about: 1. Among F-, Cl- and Br- and I-, which one has the highest polarizability? Why? 2. Among O 2 - and F-, which one has a higher polarizability? Why?

Answer to Q 1 ³ ³ F-, Cl-, Br- and I- are all belonged to the same group I- has the greatest number of electron shell The size of I- is the largest I- has the highest polarizability (more easily to be polarized)

Answer to Q 2 ³ ³ ³ O 2 - and F- have the same number of electrons The charge of O 2 - is greater than FO 2 - is easier to be polarized than F-