Inclusion of Patient Preference Information in FDA Submissions
Inclusion of Patient Preference Information in FDA Submissions Utah Life Science Summit 2016 October 6, 2016 Sharon A. Segal, Ph. D. Vice President, Technology & Regulatory Affairs Adva. Med Diana K. Salditt Regulatory Advocacy Program Director Medtronic

Agenda • Provide overview of FDA/CDRH initiatives in patient engagement and patient preference • Answer questions and provide insights on key concepts and facts related to these initiatives 2
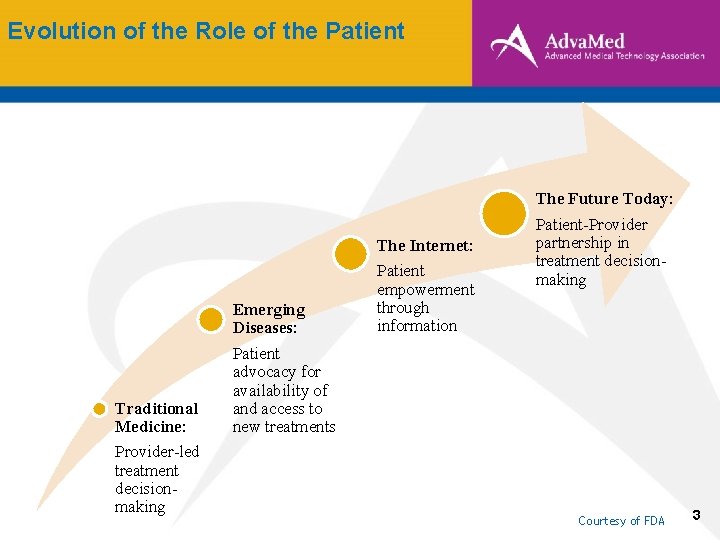
Evolution of the Role of the Patient The Future Today: The Internet: Traditional Medicine: Provider-led treatment decisionmaking Emerging Diseases: Patient advocacy for availability of and access to new treatments Patient empowerment through information Patient-Provider partnership in treatment decisionmaking Courtesy of FDA 3
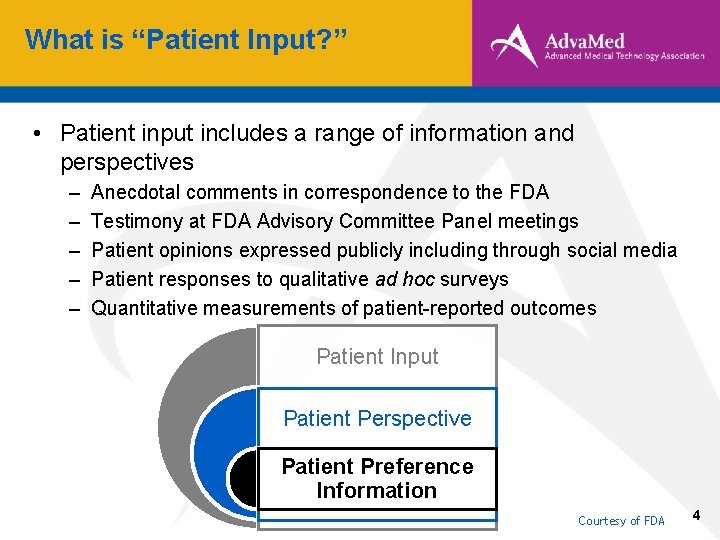
What is “Patient Input? ” • Patient input includes a range of information and perspectives – – – Anecdotal comments in correspondence to the FDA Testimony at FDA Advisory Committee Panel meetings Patient opinions expressed publicly including through social media Patient responses to qualitative ad hoc surveys Quantitative measurements of patient-reported outcomes Patient Input Patient Perspective Patient Preference Information Courtesy of FDA 4

What are “Patient Perspectives? ” • Patient perspectives refer to a type of patient input • Information relating to patients’ experiences with a disease or condition and its management • May be useful for: – better understanding the disease or condition and its impact on patients – identifying outcomes most important to patients – understanding benefit-risk tradeoffs for treatment Courtesy of FDA 5
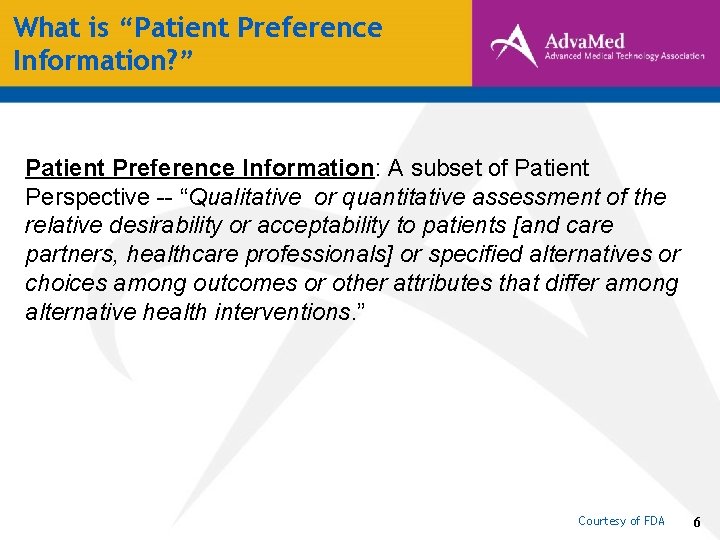
What is “Patient Preference Information? ” Patient Preference Information: A subset of Patient Perspective -- “Qualitative or quantitative assessment of the relative desirability or acceptability to patients [and care partners, healthcare professionals] or specified alternatives or choices among outcomes or other attributes that differ among alternative health interventions. ” Courtesy of FDA 6
What Can PPI Provide and How Can It Be Used? • PPI data can provide valuable information about: • • Which benefits and risks are most important to affected patients What benefit-risk tradeoffs are acceptable from the patient perspective How do these patients think about these tradeoffs Are there clinically-relevant subgroups of patients that would accept a particular benefit-risk profile and/or choose one treatment option over other alternatives • Potential Uses of PPI: • • • Inform endpoints or effect size for regulatory studies Inform subgroup considerations Labeling changes / expanded indications • Other potential uses outside regulatory context, such as shared medical decision-making. 7
Other Aspects of Patient Input • Patient-Centered Benefit-Risk: Patient views on the potential risks and benefits of specific devices; does the clinical benefit of a device outweigh its risk? • Patient Reported Outcome (PRO): Any (typically) quantitative report of the status of a patient’s health condition that comes directly from the patient, without interpretation of the patient’s response by a clinician or anyone else. – PRO Instruments are designed to measure a patient’s perceptions of health status before, during, and after therapy. – In contrast, PPI studies measure what specified type of therapy or attributes of a given therapy or diagnostic strategy a patient might prefer. 8
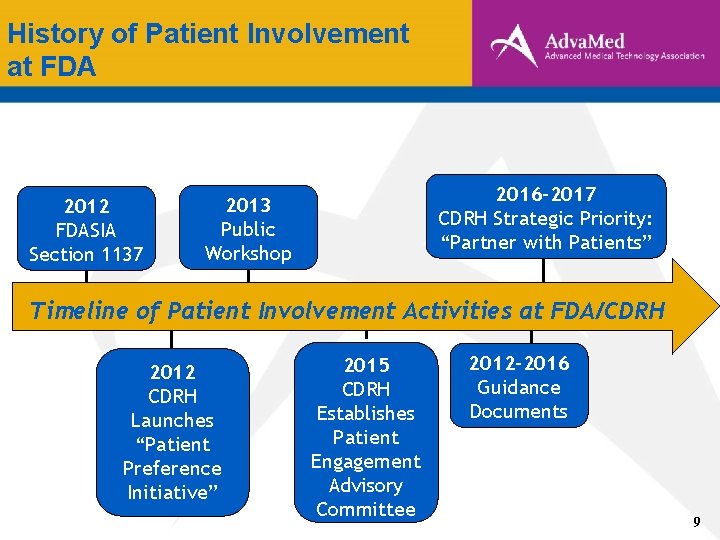
History of Patient Involvement at FDA 2012 FDASIA Section 1137 2016 -2017 CDRH Strategic Priority: “Partner with Patients” 2013 Public Workshop Timeline of Patient Involvement Activities at FDA/CDRH 2012 CDRH Launches “Patient Preference Initiative” 2015 CDRH Establishes Patient Engagement Advisory Committee 2012 -2016 Guidance Documents 9

FDASIA Section 1137 (2012) ‘‘SEC. 569 C. PATIENT PARTICIPATION IN MEDICAL PRODUCT DISCUSSION. ‘‘(a) IN GENERAL. —The Secretary shall develop and implement strategies to solicit the views of patients during the medical product development process and consider the perspectives of patients during regulatory discussions, including by— ‘ ‘(1) fostering participation of a patient representative who may serve as a special government employee in appropriate agency meetings with medical product sponsors and investigators; and ‘‘(2) exploring means to provide for identification of patient representatives who do not have any, or have minimal, financial interests in the medical products industry. ” 10

CDRH Patient Preference Initiative (2012) “The goal of the Patient Preference Initiative (PPI) is to develop a systematic way of eliciting, measuring, and incorporating patient preference information, where appropriate, into the medical device Total Product Life Cycle. Ultimately, the objective is to drive more patientcentric innovation, evaluation, and delivery to U. S. patients. As part of this initiative, CDRH seeks to advance the science of measuring patient preferences by developing guidance for industry and other stakeholders on how to assess patient valuations of benefit and risk related to relevant device types and specific illnesses and conditions. ” 11

Patient Engagement Advisory Committee (PEAC) (2015) • The Patient Engagement Advisory Committee (PEAC) was “established to ensure the needs and experiences of patients are incorporated into FDA’s work in areas such as: – Advising FDA on ways to include and foster participation of patients, where appropriate, throughout the total produce lifecycle – Advising FDA on patient perspectives about current and new approaches or policies for integrating patient input in decision making; and – Serving as a resource to FDA as a body of experts in patient experience, needs, and the activities of the patient community. ” 12

CDRH Patient Engagement Advisory Committee (2015) (cont’d) • Mission of PEAC: Provide advice to FDA, on complex issues relating to medical devices, the regulation of devices, and their use by patients. • PEAC may consider topics such as: – Agency guidance and policies – clinical trial or registry design – patient preference study design – benefit-risk determinations – device labeling – unmet clinical needs – available alternatives – patient reported outcomes and device-related quality of life or health status issues – other patient-related topics. 13

CDRH Patient Engagement Advisory Committee (2015) (cont’d) PEAC composed of nine core members who are knowledgeable in the following areas: • clinical research • primary care patient experience • health care needs of patient groups in the United States. • patient and health professional organizations • methodologies for eliciting patient preferences • strategies for communicating benefits, risks and clinical outcomes to patients and research subjects. 14

CDRH Patient Engagement Advisory Committee (2015) (cont’d) PEAC duties include discussing and providing advice and recommendation in ways such as: • Identifying new approaches • promoting innovation • recognizing unforeseen risks or barriers • identifying unintended consequences that could result from FDA policy. 15

CDRH 2016 -2017 Strategic Priorities “Partner with Patients” • “We believe that if CDRH is to successfully achieve a mission and vision in the service of patients, we must interact with patients as partners and work together to advance the development and evaluation of innovative devices, and monitor the performance of marketed devices. ” Goals: • “Promote a culture of meaningful patient engagement by facilitating CDRH interaction with patients • Increase use and transparency of patient input as evidence in our Decision making” 16

Medical Device Patient/Benefit-Risk Guidance Documents 17
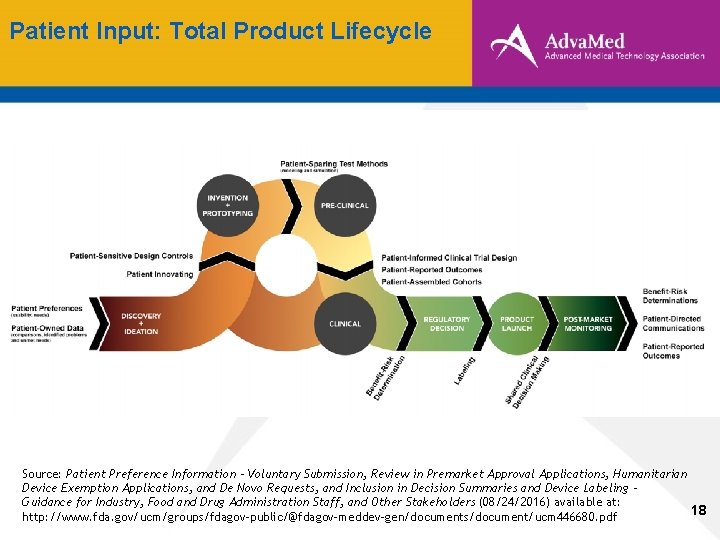
Patient Input: Total Product Lifecycle Source: Patient Preference Information - Voluntary Submission, Review in Premarket Approval Applications, Humanitarian Device Exemption Applications, and De Novo Requests, and Inclusion in Decision Summaries and Device Labeling Guidance for Industry, Food and Drug Administration Staff, and Other Stakeholders (08/24/2016) available at: 18 http: //www. fda. gov/ucm/groups/fdagov-public/@fdagov-meddev-gen/documents/document/ucm 446680. pdf

Overview of PPI Guidance • Objectives/goals: – Encourage submission of PPI, if available, by sponsors or other stakeholders to FDA and to aid in FDA decision-making; – Outline recommended qualities of patient preference studies, which may result in valid scientific evidence; – Provide recommendations for collecting and submitting PPI to FDA; and – Discuss FDA’s inclusion of PPI in its decision summaries and provide recommendations for inclusion of such information in device labeling 19

Overview of PPI Guidance • Important caveats: – Submission of PPI information in marketing applications is VOLUNTARY – PPI may not be relevant or appropriate for all device types – Review standards for PMAs, HDEs, and De Novos are not changed 20
Scope/Use of PPI Guidance Document • PPI can play a role in FDA’s benefit-risk assessment for certain devices in several major ways, including: – Identify the most important benefits and risks of a technology from a patient’s perspective; – Assess the relative importance to patients of different attributes of benefit and risk, and the tradeoffs of these benefits and risks for a given technology; and – Understand the heterogeneity or distribution of patient preferences regarding benefits and risks of various treatment or diagnostic options. – Include PPI in FDA decision summaries and provide recommendations for the inclusion of such information in labeling for certain devices 21

Device Types for which PPI May be Useful • Devices with a direct patient interface • Devices intended to yield significant health and appearance benefits. • Devices intended to directly affect health-related quality of life • Certain life-saving but high-risk devices • Devices developed to fill an unmet medical need or treat a rare disease or condition. • Devices that offer alternative benefits to those already marketed • Devices with novel technology 22
Role of PPI in Decision-Making at FDA • Patients’ preferences vary: Some patients may be willing to accept higher risks to potentially achieve a small benefit, whereas others may be more risk averse, requiring more benefit to be willing to accept certain risks. • Valid Scientific Evidence: Patient-centered variations in tolerance to risks and perspective on benefits may, in the aggregate, reveal a population-level assessment of benefitrisk that may be considered valid scientific evidence and may inform FDA’s benefit-risk assessment for a device. 23

Role of PPI in Decision-Making at FDA • Use of device in a subset: Can be approved if valid scientific evidence shows that the requisite statutory standard is met for use of a device in a subset of the population for which an indication is requested • Conditions of Approval: When “patient preference studies [may] help sponsors and FDA identify a subset of patients for whom the benefits outweigh the risks, ” conditions of approval may be imposed, such as: – such as shared decision-making tools, – specialized patient labeling, – postapproval studies to mitigate potential risk. 24
When PPI is Less Valuable • When the patient is not a major stakeholder or decisionmaker • When the disease state/ technology are generally understood by sponsors and FDA staff and there is significant regulatory precedent for approval • When benefits are high and risks are low • When the treatment is clearly superior to existing therapies with no tradeoffs in risk • When the treatment meets an unmet medical need with poor outcomes such that the risk of treatment will not be greater than the risks of the untreated disease Section III of the MDIC Framework report (mdic. org/pcbr/framework-pdf/) 25
Examples of the Use of PPI in Decision. Making for Marketing Applications • Probable benefit outweighs probable risk for a subset of patients • Patient preference information helps inform FDA reviewer considerations • Increased risk and similar effectiveness in comparison to alternatives but clear patient preference for certain device attributes 26
Methods to Evaluate Patient Preference Variety of methods available; active and evolving space • Qualitative PPI may “be useful in identifying which outcomes, endpoints, or other attributes are valued most by patients and which factors affect patients’ perspectives on risk and benefit. • Quantitative PPI can “provide estimates of how much different outcomes, endpoints, or other attributes are valued by patients, and the tradeoffs that patients state or demonstrate they are wiling to make among them. ” They ensure that different outcomes are “properly weighed in the same scale and therefore can be compared. ” • Early interaction with the relevant review division at FDA is crucial when developing clinical testing protocols that include PPI. 27
Recommended Qualities of Patient Preference Studies Patient Centeredness • Patients are the focus of the study • Studies should measure the preferences and perspectives of well-informed patients Representativeness of the Sample and Generalizability of Results • Studies should measure the preferences of a representative sample of adequate size so that the study results can be reasonably generalized to the population of interest Capturing Heterogeneity of Patients’ Preferences • Patients’ benefit-risk tradeoff preferences may be heterogeneous even among those with the same disease or condition • Studies should reflect the preferences of patients from the full spectrum of disease for which the device is intended to be used Comprehension by Study Participants • Ensure that study participants fully understand the harm, risk, benefit, uncertainty, and other medical information being communicated to them Courtesy of FDA 28

Recommended Study Qualities: Good Study Design Established Good Research Practices by Recognized Professional Organizations • Quality of a study may be established if it follows guidelines for good research practices established by a recognized professional organization Effective Communication of Benefit, Harm, Risk, and Uncertainty • Reduce uncertainty caused by health numeracy • Example 1: Avoid solely verbal descriptions of uncertainty; Use multiple formats simultaneously • Example 2: Pretest the communication format Minimal Cognitive Bias • Minimize cognitive biases such as framing, anchoring, simplifying heuristics, or ordering effect Relevance • Inclusion and omission of harm, risk, benefit, and uncertainty should be well justified • Useful to ensure some consistency among the benefits, harms, risks and other attributes • Relevance of specific endpoints to potential clinical outcomes should be clearly Courtesy of FDA 29 communicated to patients to properly elicit preference

Recommended Study Qualities Study Conduct and Analysis Study Conduct • Compliance of research staff and study participants with the study protocol Logical Soundness • Data should include internal-validity tests of logic and consistency • Verified for conformity with logic and consistency Robustness of Analysis of Results • Sources of uncertainty • Sensitivity analysis Courtesy of FDA 30

Inclusion of PPI in Decision Summaries and Device Labeling • When FDA considers patient preference studies in its consideration of a premarket submission, such studies generally are included in the decision summary • Inclusion of PPI in FDA’s public decision summaries can be helpful to health care professionals and patients in making health care decisions involving difficult benefit-risk tradeoffs or novel treatments • PPI that is reviewed by FDA and supports FDA’s approval or marketing authorization should also be described in the device labeling – It is important for the device product labeling to contain sufficient information about the benefits and risks of the treatment and diagnostic options under consideration (Please see FDA Guidance: Labeling – Regulatory Requirements for Medical Devices (FDA 89 -4203). Courtesy of FDA 31

MDIC Patient-Centered Benefit-Risk (http: //mdic. org/pcbr/) Undertaken to improve ability to include patient perspectives in the development, premarket approval, and post-market evaluation of medical devices. • Find scientifically valid ways to reliably assess patient views on the potential risks and benefits of specific devices. • Establish a credible framework for incorporating that information into device development and benefit-risk assessments. 32

MDIC Patient-Centered Benefit-Risk (http: //mdic. org/pcbr/) Work completed thus far: • A framework for incorporating information on those preferences into benefit-risk assessments of new medical technology. • A catalog of methods that can be used to assess patient preferences about the benefits and risks of a medical technology. • An analysis of gaps in current methods for assessing patient preferences. • Frequently asked questions from sponsors about patient preference studies): http: //mdic. org/pcbr/resources/ • An agenda for further research. 33

Thank you! 34
- Slides: 34