Inclisiran ORION Program Update Dr David Kallend Previously







- Slides: 17

Inclisiran ORION Program Update Dr David Kallend Previously Chief Medical Officer The Medicines Company CRT, February 2020

Kallend, David MBBS (LON) Employment and Salary: The Medicines Company Stock options: The Medicines Company

Mechanism of action si. RNA 3

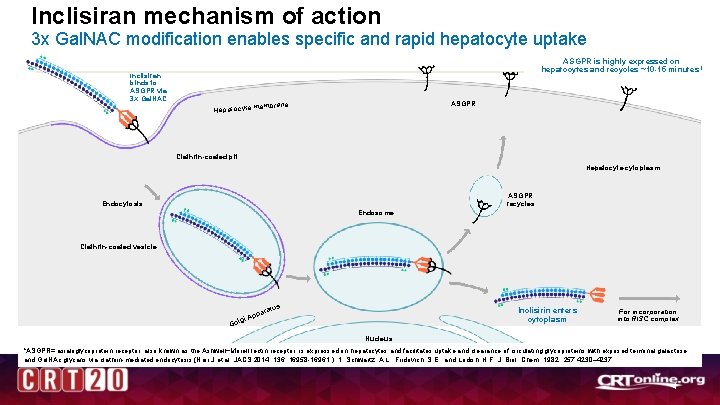
Inclisiran mechanism of action 3 x Gal. NAC modification enables specific and rapid hepatocyte uptake Inclisiran binds to ASGPR via 3 X Gal. NAC ASGPR is highly expressed on hepatocytes and recycles ~10 -15 minutes 1 ASGPR membrane Hepatocyte Clathrin-coated pit Hepatocyte cytoplasm ASGPR recycles Endocytosis Endosome Clathrin-coated vesicle s ratu ppa gi A Gol Inclisirin enters cytoplasm For incorporation into RISC complex Nucleus *ASGPR= asialoglycoprotein receptor, also known as the Ashwell−Morell lectin receptor, is expressed on hepatocytes and facilitates uptake and clearance of circulating glycoproteins with exposed terminal galactose and Gal. NAc glycans via clathrin-mediated endocytosis (Nair J et al. JACS 2014; 136: 16958 -16961. ). 1. Schwartz, A. L. , Fridovich, S. E. , and Lodish, H. F. J. Biol. Chem. 1982; 257: 4230– 4237 4

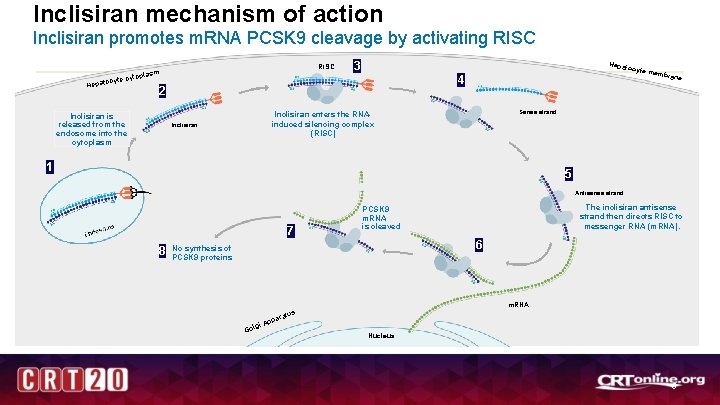
Inclisiran mechanism of action Inclisiran promotes m. RNA PCSK 9 cleavage by activating RISC c Hepato RISC sm topla yte cy 3 Hepato 2 Inclisiran is released from the endosome into the cytoplasm cyte m 4 ne Sense strand Inclisiran enters the RNAinduced silencing complex (RISC) Inclisiran embra 1 5 Antisense strand 7 8 The inclisiran antisense strand then directs RISC to messenger RNA (m. RNA). PCSK 9 m. RNA is cleaved 6 No synthesis of PCSK 9 proteins m. RNA s p gi A Gol atu par Nucleus 5

Phase II Foundations ORION-1 ORION-3 6

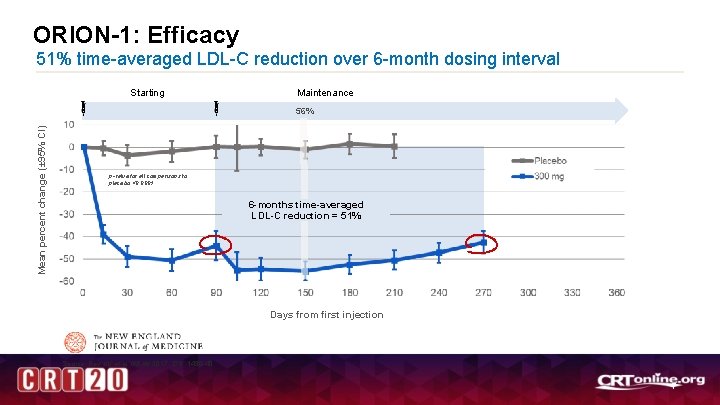
ORION-1: Efficacy 51% time-averaged LDL-C reduction over 6 -month dosing interval Starting Maintenance Mean percent change (± 95% CI) 56% p-value for all comparisons to placebo <0. 0001 6 -months time-averaged LDL-C reduction = 51% Days from first injection Source: Ray KK et al. NEJM 2017; 376: 1430 -40. 7

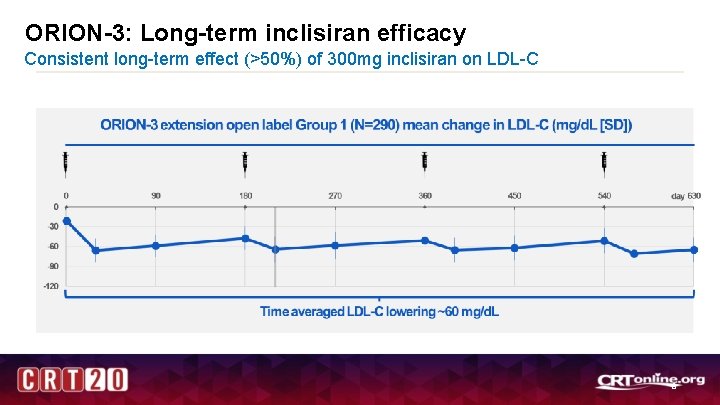
ORION-3: Long-term inclisiran efficacy Consistent long-term effect (>50%) of 300 mg inclisiran on LDL-C 8

Phase III Efficacy and Safety ORION-9 ORION-10 ORION-11 Confidential 9

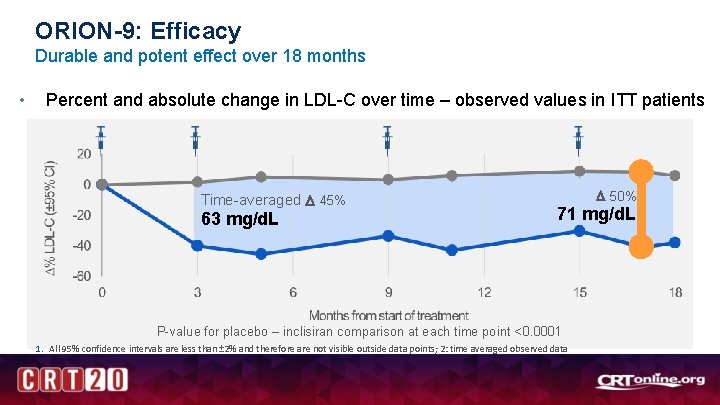
ORION-9: Efficacy Durable and potent effect over 18 months • Percent and absolute change in LDL-C over time – observed values in ITT patients Time-averaged 45% 63 mg/d. L 50% 71 mg/d. L P-value for placebo – inclisiran comparison at each time point <0. 0001 1. All 95% confidence intervals are less than ± 2% and therefore are not visible outside data points; 2: time averaged observed data

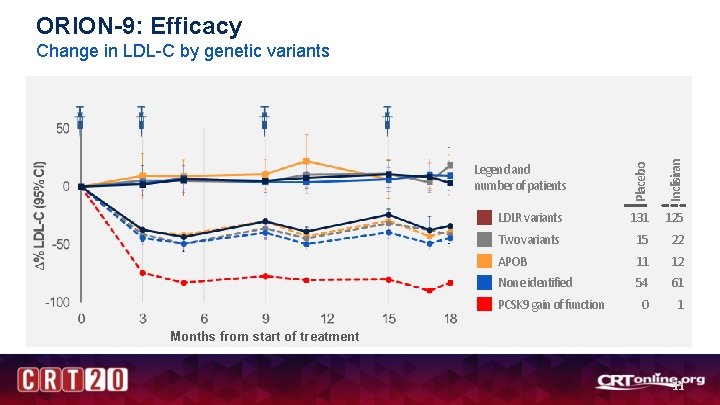
ORION-9: Efficacy Legend and number of patients Placebo Inclisiran Change in LDL-C by genetic variants LDLR variants 131 125 Two variants 15 22 APOB 11 12 None identified 54 61 0 1 PCSK 9 gain of function Months from start of treatment 11

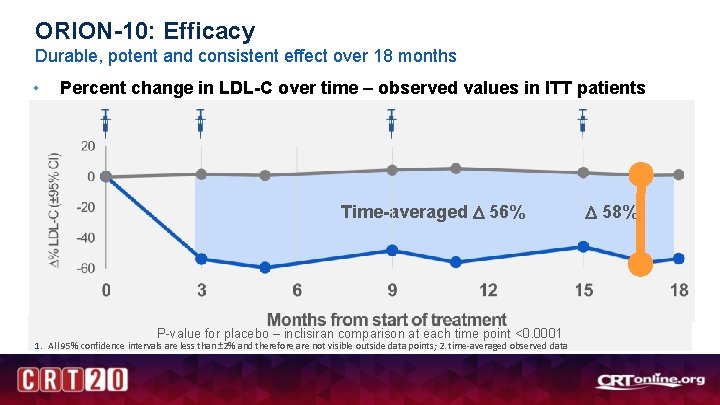
ORION-10: Efficacy Durable, potent and consistent effect over 18 months • Percent change in LDL-C over time – observed values in ITT patients Time-averaged 56% P-value for placebo – inclisiran comparison at each time point <0. 0001 1. All 95% confidence intervals are less than ± 2% and therefore are not visible outside data points; 2. time-averaged observed data 58%

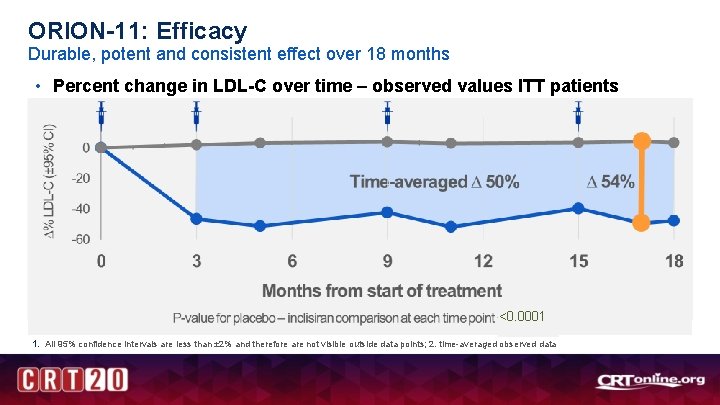
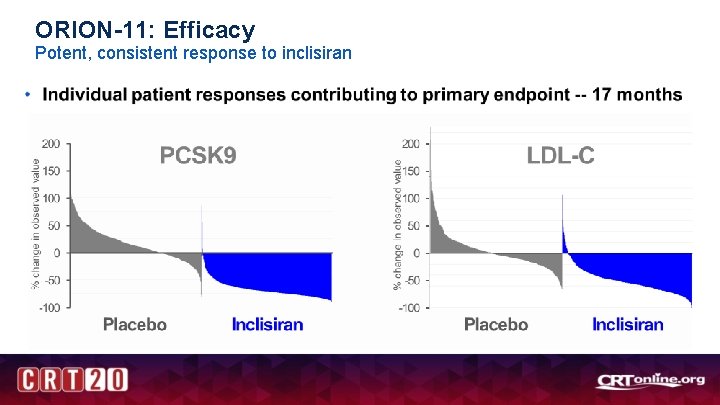
ORION-11: Efficacy Durable, potent and consistent effect over 18 months • Percent change in LDL-C over time – observed values ITT patients <0. 0001 1. All 95% confidence intervals are less than ± 2% and therefore are not visible outside data points; 2. time-averaged observed data

ORION-11: Efficacy Potent, consistent response to inclisiran

Safety Summary • No difference from placebo for any laboratory parameter including hepatic, renal, hematology and muscle biomarkers • Adverse event profile generally similar to placebo • No imbalance in deaths or malignancy • Only adverse event considered to be related to administration of inclisiran are injection site reactions. These are predominantly mild and transient. • Overall highly reassuring safety profile in a high cardiovascular risk population Very high safety margin demonstrated in non-clinical studies

Conclusions and implications Inclisiran is the first cholesterol lowering si. RNA • Inclisiran achieves durable and potent LDL-C reduction with only 2 x yearly injection • Same dose and regimen for all patient populations e. g. renal and hepatic impairment, Ho. FH • Excellent safety profile in a high cardiovascular risk population • Administration by HCP potentially coincides with typical six-monthly patient visits – Lends itself to routine clinical practice – Enables health care provider control over medication adherence

Thank you