In hydrogen all orbitals in the same shell
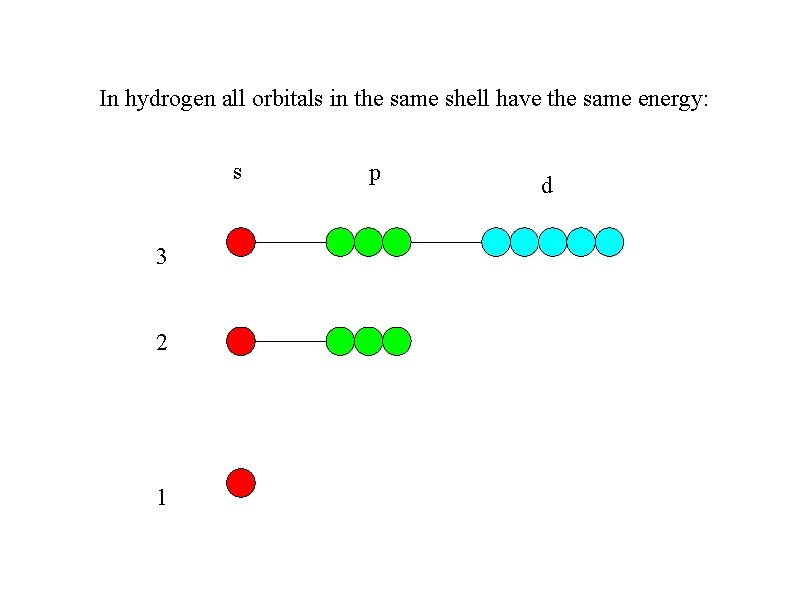
In hydrogen all orbitals in the same shell have the same energy: s 3 2 1 p d
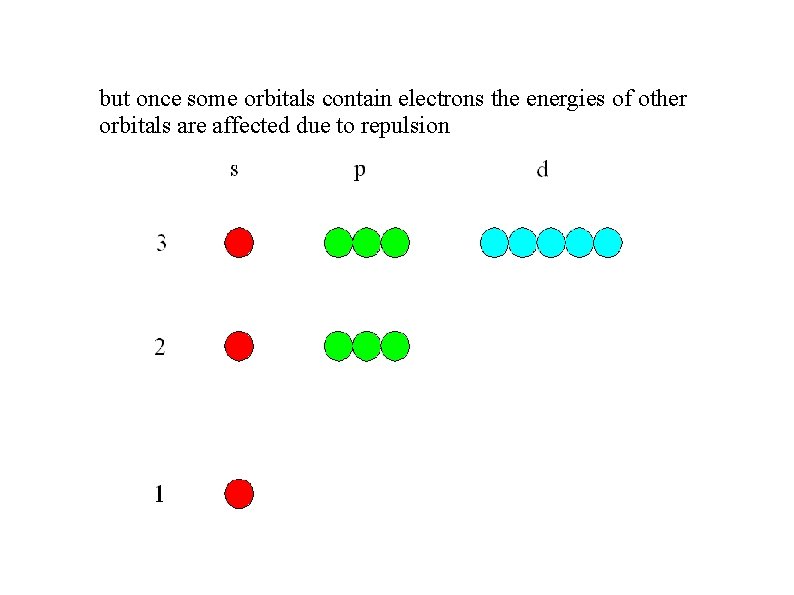
but once some orbitals contain electrons the energies of other orbitals are affected due to repulsion

so the p and d orbitals have higher energy than the s orbitals of the same shell: s 3 p d 2 You need to copy this diagram 1
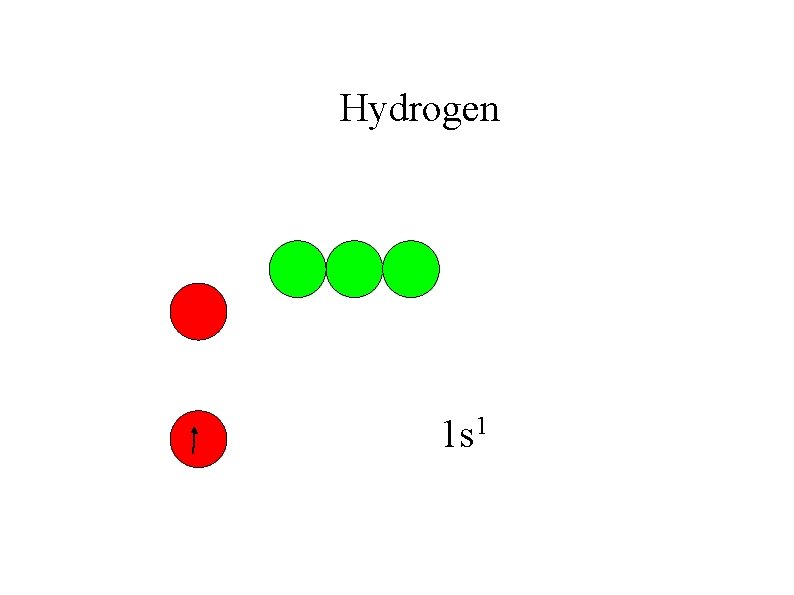
Hydrogen 1 s 1
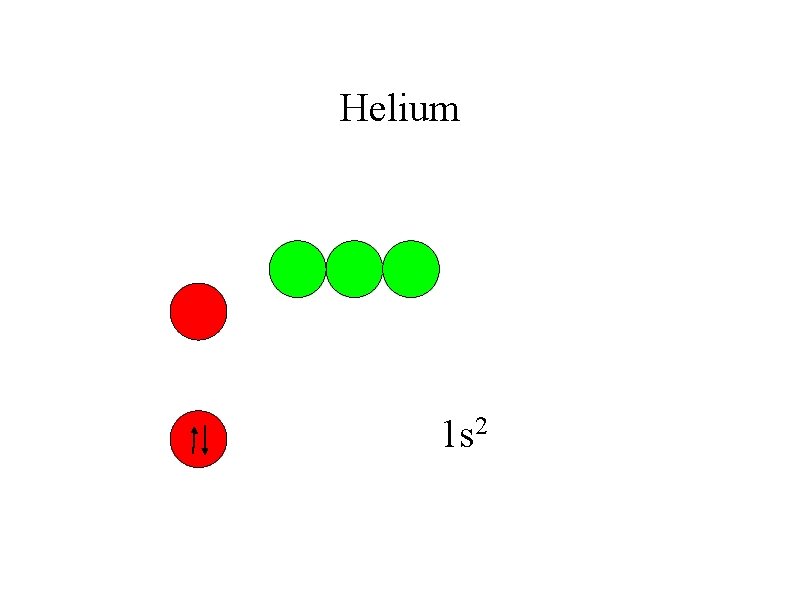
Helium 1 s 2
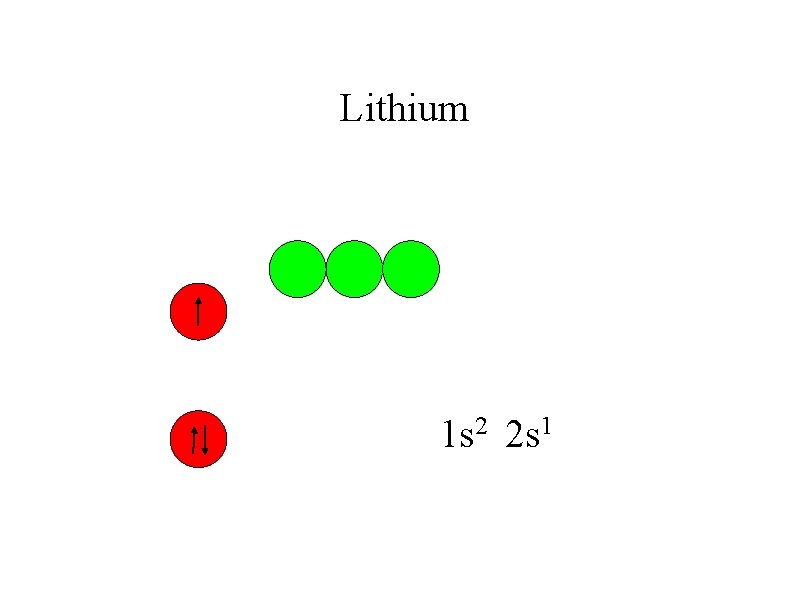
Lithium 1 s 2 2 s 1
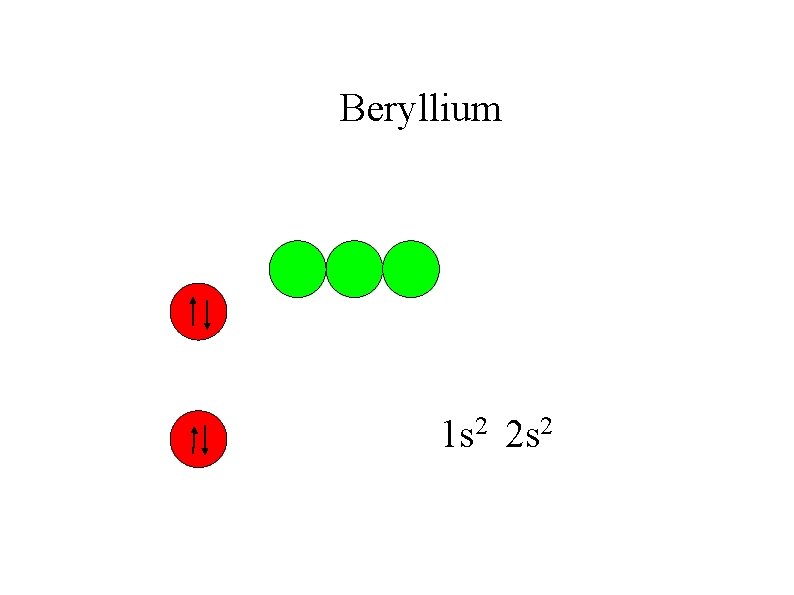
Beryllium 1 s 2 2 s 2
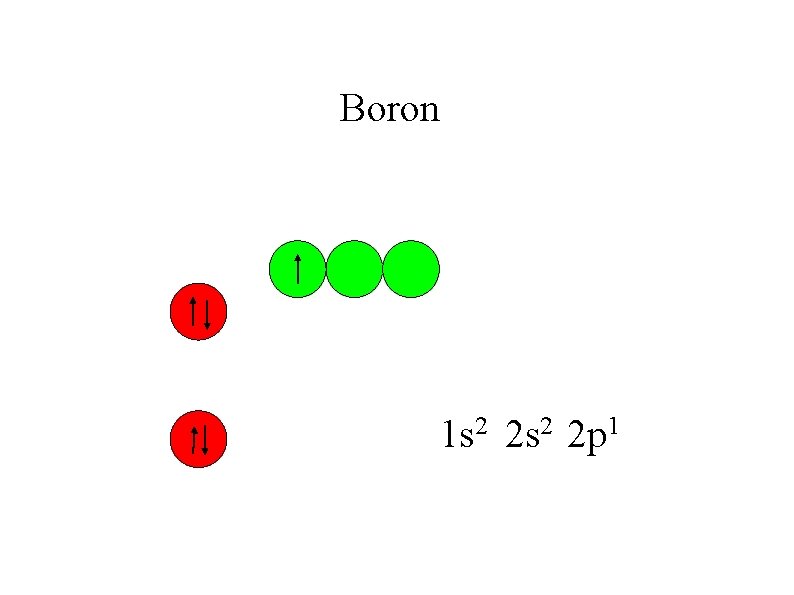
Boron 1 s 2 2 p 1
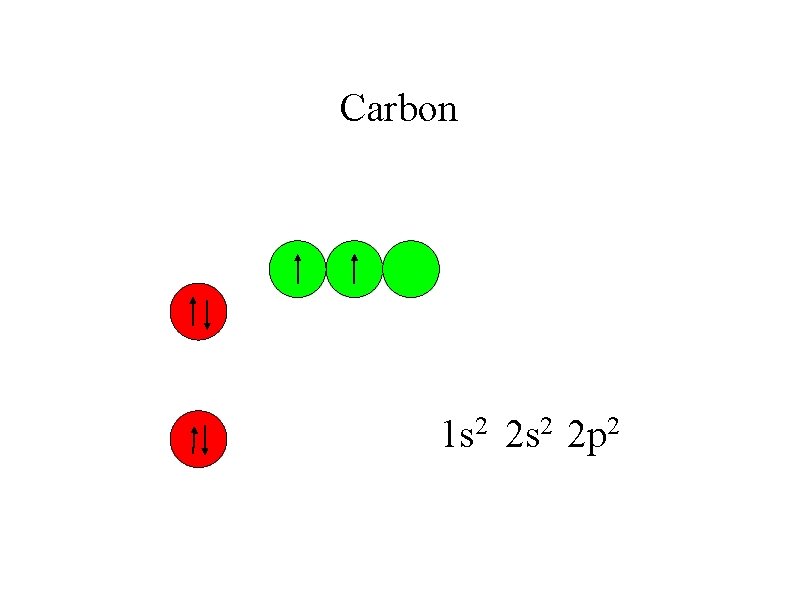
Carbon 1 s 2 2 p 2
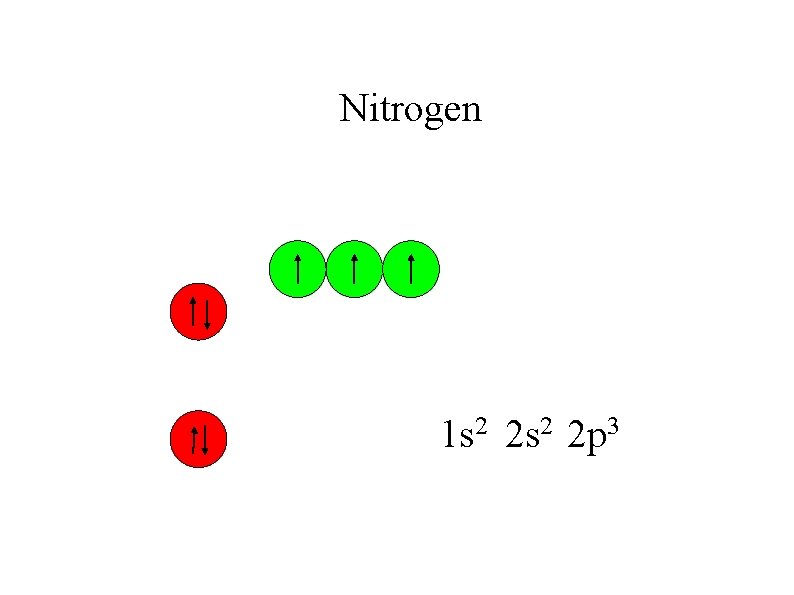
Nitrogen 1 s 2 2 p 3
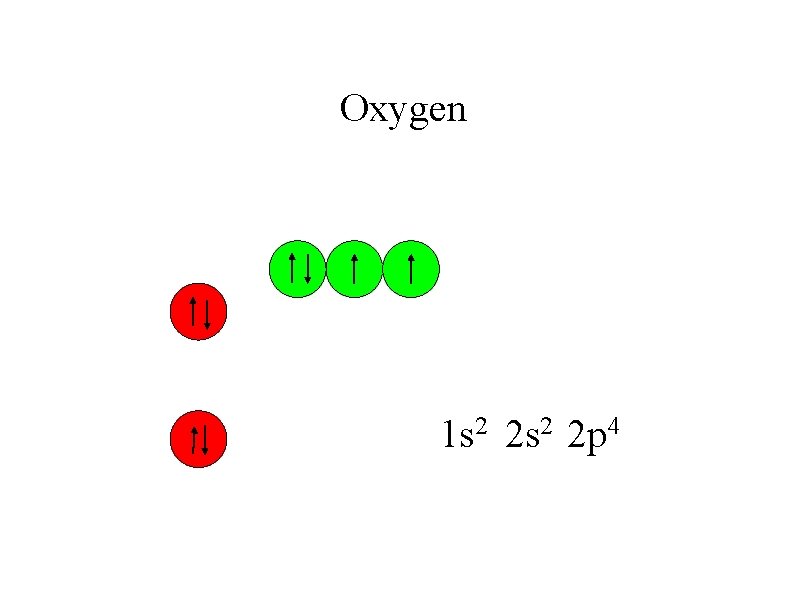
Oxygen 1 s 2 2 p 4
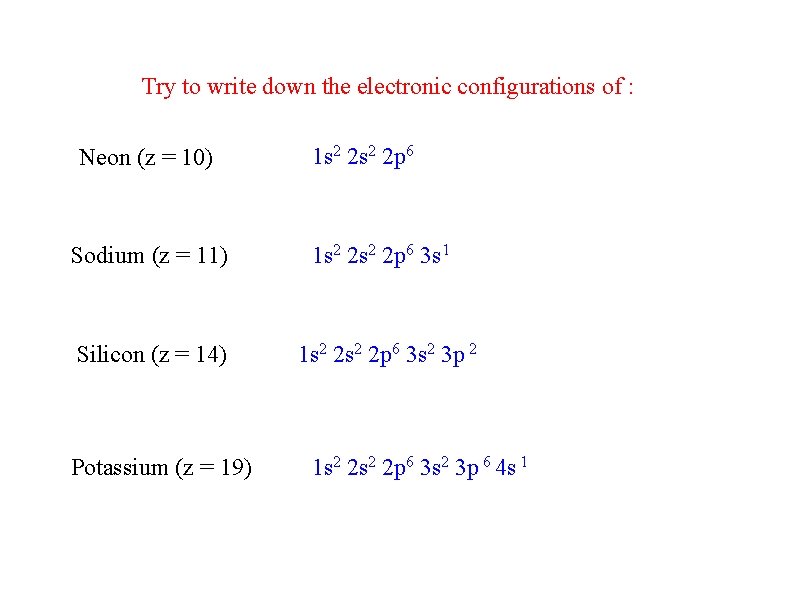
Try to write down the electronic configurations of : Neon (z = 10) 1 s 2 2 p 6 Sodium (z = 11) 1 s 2 2 p 6 3 s 1 Silicon (z = 14) 1 s 2 2 p 6 3 s 2 3 p 2 Potassium (z = 19) 1 s 2 2 p 6 3 s 2 3 p 6 4 s 1
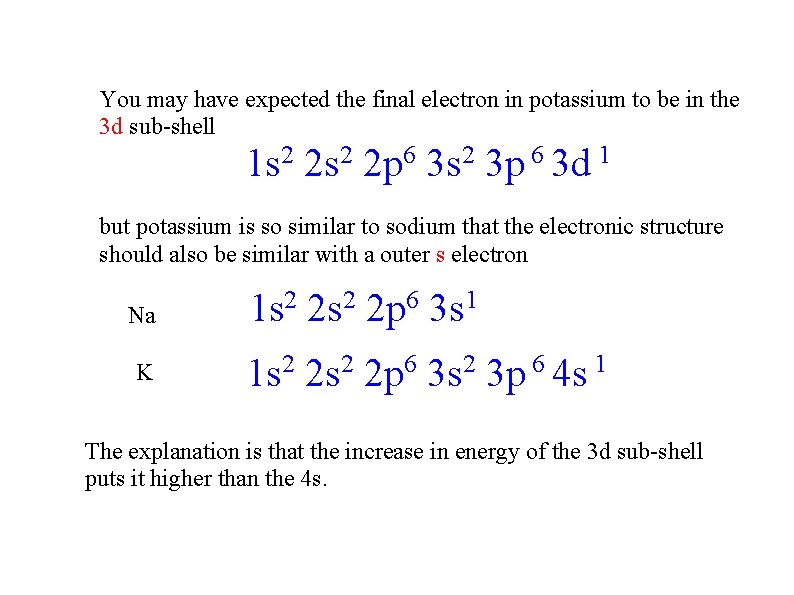
You may have expected the final electron in potassium to be in the 3 d sub-shell 2 1 s 2 2 s 6 2 p 2 3 s 6 1 3 p 3 d but potassium is so similar to sodium that the electronic structure should also be similar with a outer s electron Na K 2 1 s 2 2 s 6 2 p 1 3 s 1 s 2 2 p 6 3 s 2 3 p 6 4 s 1 The explanation is that the increase in energy of the 3 d sub-shell puts it higher than the 4 s.
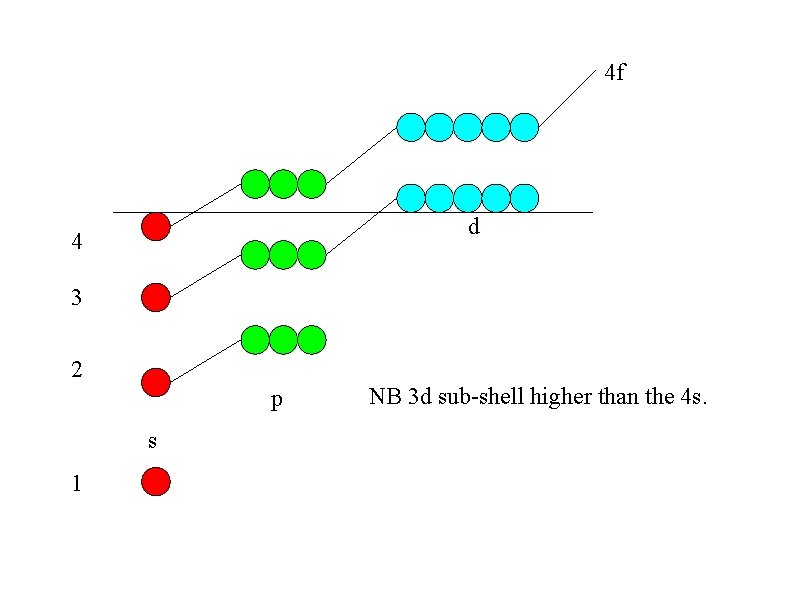
4 f d 4 3 2 p s 1 NB 3 d sub-shell higher than the 4 s.
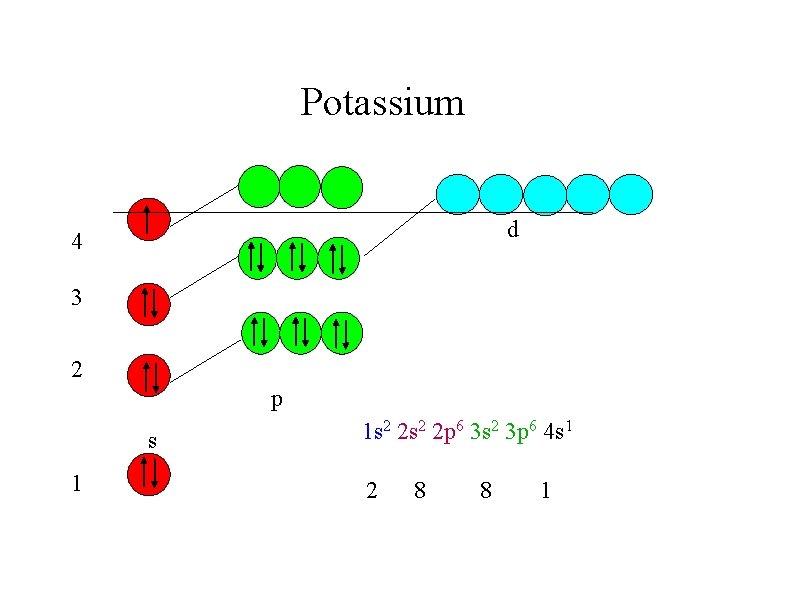
Potassium d 4 3 2 p s 1 1 s 2 2 p 6 3 s 2 3 p 6 4 s 1 2 8 8 1
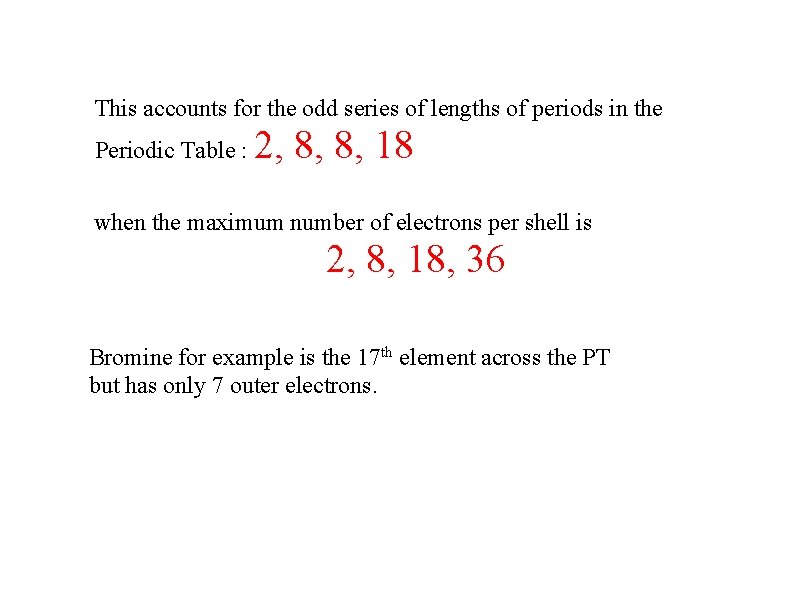
This accounts for the odd series of lengths of periods in the Periodic Table : 2, 8, 8, 18 when the maximum number of electrons per shell is 2, 8, 18, 36 Bromine for example is the 17 th element across the PT but has only 7 outer electrons.
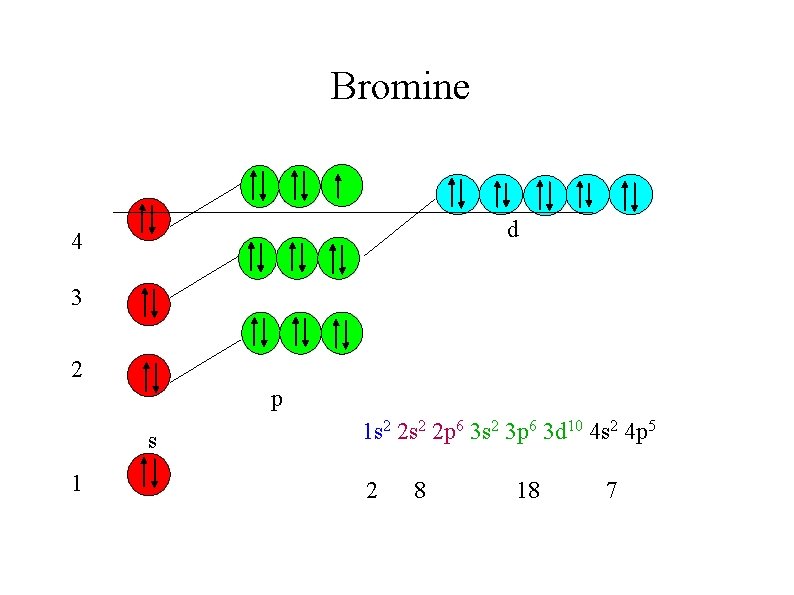
Bromine d 4 3 2 p s 1 1 s 2 2 p 6 3 s 2 3 p 6 3 d 10 4 s 2 4 p 5 2 8 18 7
- Slides: 17