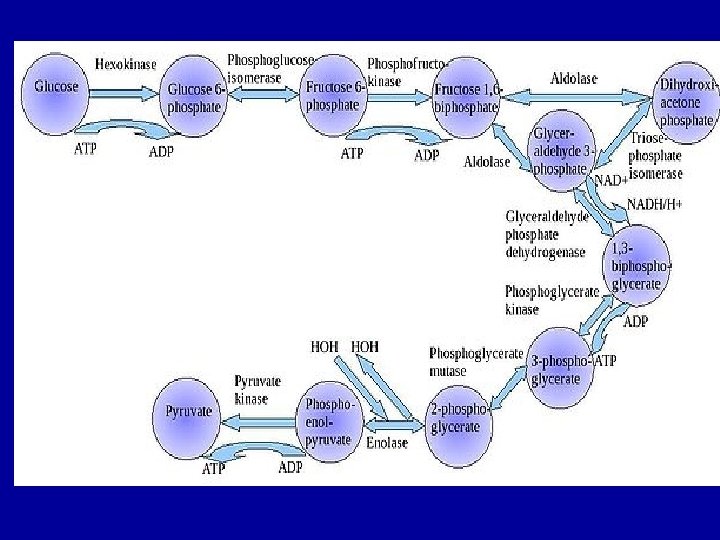
In glycolysis a molecule of glucose is degraded
























- Slides: 57


- In glycolysis a molecule of glucose is degraded in a series of enzyme-catalyzed reactions to yeild 2 molecules of pyruvate. The three irreversible enzymecatalyzed reactions are: 1) Hexokinase (or glucokinase) 2) Phosphofructokinase 3) Pyruvate kinase Pyruvate then passes into the mitochondrion and enter krebs cycle

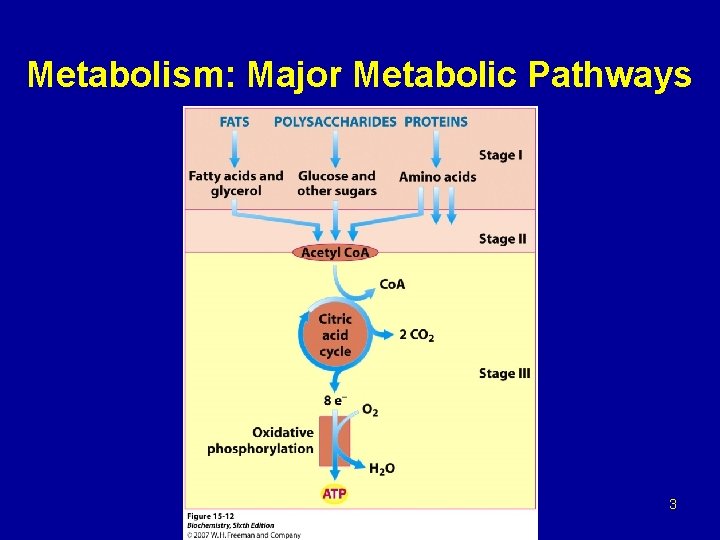
Metabolism: Major Metabolic Pathways 3

Hexokinase - Mammals have several isozymes of hexokinase that catalyze the phosphorylation of glucose to glucose 6 -phosphate - In most tissues the phosphorylation of glucose is catalyzed by hexokinase, that is one of the three regulatory enzymes of glycolysis. It has a broad specificity and is able to phosphorylate several hexoses in addition to glycosis. - Hexokinase is inhibited by the reaction product, glycose 6 -phosphate, that accumulates when further metabolism of this hexose phosphate is reduced, for example by a high ATP/ADP ratio. - Hexokinase has a high affinity for glucose. This permits efficient phosphorylation and subsequent metabolism of glucose even when tissue concentration of glucose are low.

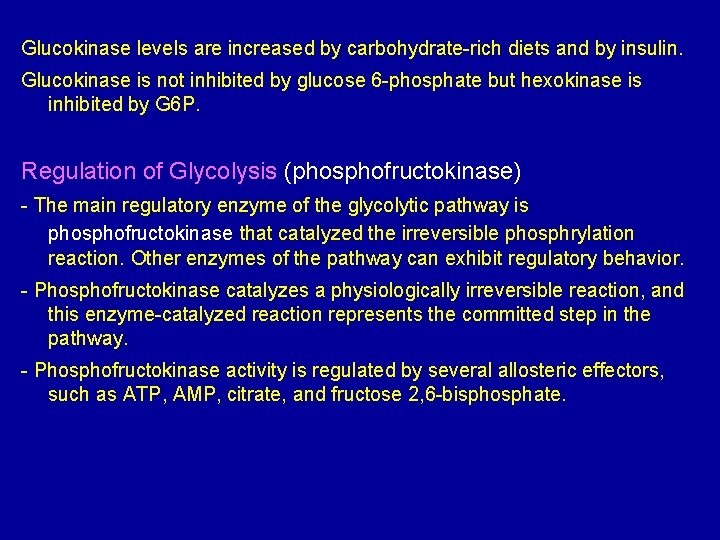
Glucokinase occur in the liver and B-cells of the pancreas. It is the predominant enzyme for the phosphorylation of glucose. Glucokinase differs from hexokinase in requiring a much higher glucose concentration for half saturation. Therefore, glucokinase function only when the intracellular concentration of glucose in the hepatocyte is elevated such as during the brief period following consumption of a carbohydrate-rich meal, when high levels of glucose are delivered to the liver via portal vein. Glucokinase has a high Vmax allowing the liver to effectively remove this flood of glucose from the portal blood. This prevents large amount of glucose from entering the systemic circulation and thus minimizes hyperglycemia during the absorptive period.

Glucokinase levels are increased by carbohydrate-rich diets and by insulin. Glucokinase is not inhibited by glucose 6 -phosphate but hexokinase is inhibited by G 6 P. Regulation of Glycolysis (phosphofructokinase) - The main regulatory enzyme of the glycolytic pathway is phosphofructokinase that catalyzed the irreversible phosphrylation reaction. Other enzymes of the pathway can exhibit regulatory behavior. - Phosphofructokinase catalyzes a physiologically irreversible reaction, and this enzyme-catalyzed reaction represents the committed step in the pathway. - Phosphofructokinase activity is regulated by several allosteric effectors, such as ATP, AMP, citrate, and fructose 2, 6 -bisphosphate.

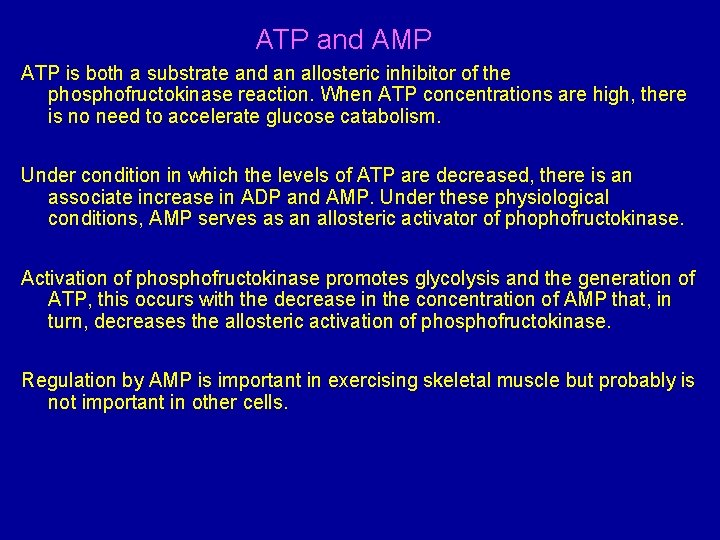
ATP and AMP ATP is both a substrate and an allosteric inhibitor of the phosphofructokinase reaction. When ATP concentrations are high, there is no need to accelerate glucose catabolism. Under condition in which the levels of ATP are decreased, there is an associate increase in ADP and AMP. Under these physiological conditions, AMP serves as an allosteric activator of phophofructokinase. Activation of phosphofructokinase promotes glycolysis and the generation of ATP, this occurs with the decrease in the concentration of AMP that, in turn, decreases the allosteric activation of phosphofructokinase. Regulation by AMP is important in exercising skeletal muscle but probably is not important in other cells.

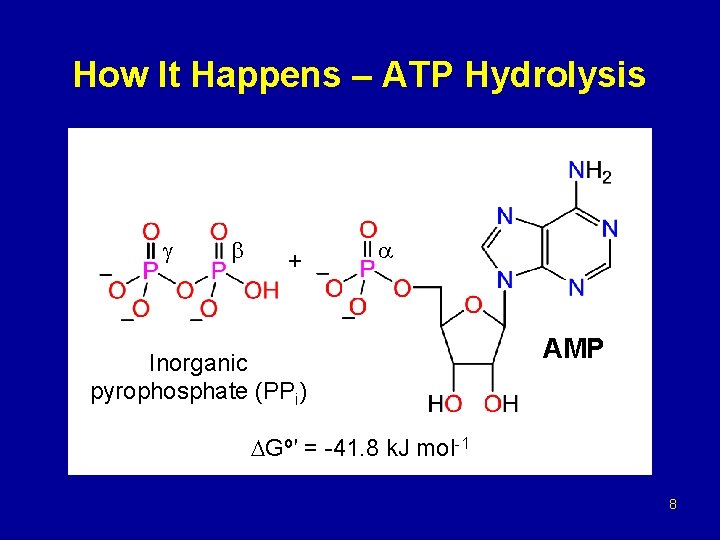
How It Happens – ATP Hydrolysis + Inorganic pyrophosphate (PPi) AMP Gº′ = -41. 8 k. J mol-1 8

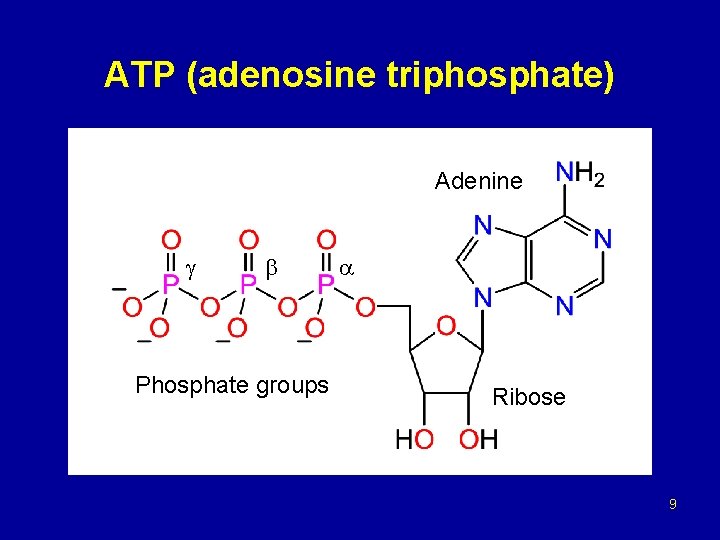
ATP (adenosine triphosphate) Adenine Phosphate groups Ribose 9

Formation of pyruvate The conversion of phosphoenolpyruvate (PEP) to pyruvate is catalyzed by pyruvate kinase, the third irreversible reaction of glycolysis In liver, pyruvate kinase is activated by fructose 1, 6 -bisphosphate, the product of the phosphofructokinase reaction. Phosphorylation by c. AMP-dependent protein kinase leads to inactivation of pyruvate kinase in the liver When blood glucose levels are low, elevated glucagons thus increases the intracellular level of c. AMP, that favors the phosphorylation and inactivation of pyruvate kinase

Carboxylation of pyruvate - The first step in the synthesis of glucose from pyruvate is the irreversible conversion of pyruvate to phosphoenolpyruvate (PEP) by pyruvate kinase. - In gluconeogenesis, pyruvate is first carboxylated by pyruvate carboxylase to oxaloacetate (OAA), that is then converted to PEP by the action of PEP-carboxykinase [Note that pyruvate carboxylase is also found in the mitochondria of liver and kidney cells, but not of muscle].

- Several vitamins provide cofactors for the enzymes involved in these metabolic pathways. E. g. the pyruvate dehydrogenase reaction need cofactors derived from thiamine, riboflavin, lipoic acid and pantothenic acid a deficiency of any of these could cause malfunctioning of a metabolic pathway at the particular enzymetic reaction where the cofactor is involved.

gluconeogenesis provides a substantial fraction of the glucose produced in fasting human even after a few hours fast. Gluconeogenesis occurs in liver and to a smaller extent in kidney. The noncarbohydrate precursors that can be converted to glucose include the glycolysis products lactate and pyruvate, citric acid cycle intermediates, and the carbon skeletons of most amino acids. However, all these substances must be converted to oxaloacetate, the starting material for gluconeogenesis.

Citric acid cycle TCA (Krebs cycle) - TCA plays several roles in metabolism, its central function is the oxidation of acetyl Co. A to CO 2 and H 2 O. - Acetyl Co. A is derived from the metabolism of fuel molecule such as amino acids, fatty acids, and carbohydrates. - The cycle occurs totally in the mitochondrial matrix and is therefore in close proximity to reactions of oxidative phosphorylation.

Regulation of the citric acid cycle - The pyruvate dehydrogenase complex that consist of multiple copies of each of the three enzymes: Pyruvate dehydrogenase (E 1) Dihydrlipoyl transacetylase (E 2) Dihydrolipoyl dehydrogenase (E 3) - Is regulated both allosterically and by covalent modification. The compex is strongly inhibited by ATP, as well as by acetyl-Co. A and NADH, the products of the reaction.

- The allosteric inhibition of pyruvate oxidation is greatly enhanced when long-chain fatty acids are available. - AMP, Co. A, and NAD+, all of which accumulate when too little acetate flows into TCA cycle, allosterically activate the pyruvate dehydrogenase complex. - Therefore this enzyme activity turned off when ample fuel is available in the form of fatty acids and acetyl. Co. A and when the cell’s ATP concentration and [NADH]/[NAD+] ratio are high. - And turned on when energy demands are high and greater flux of acetyl-Co. A into the TCA cycle is requierd.

In the pyruvate dehydrogenase complex these allosteric regulation mechanisms are complemented by a second level of regulation, covalent protein modification. Covalent modification: The pyruvate dehydrogenase complex exists in two forms: an active, nonphosphorylated form and an inactive, phophorylated form. Phophorylated and nonphosphorylated can be intercinverted by two separate enzymes, a kinase and a phosphatase. The kinase is activated by an increase in the ratio of acetyl. Co. A/Co. A or NADH/NAD+.

- An increase in the ratio of ADP/ATP, which signals increased demand for energy production, inhibits the kinase and allows the phosphatase to produce more of the active, nonphosphorylated enzyme. Regulation of the TCA cycle by activation and inhibition of enzyme activities - In contrast to glycolysis, which is regulated primarily by phosphofructokinase, the TCA cycle is controlled by regulation of several enzyme activities. - The most important of these regulated enzymes are citrate synthase, isocitrate dehydrogenase, and alpha-ketoglutarate dehydrogenase complex

Regulation by the availability of ADP: 1 - effects of elevated ADP: - Energy consumption due to muscular contraction, biosynthetic reactions, or other processes result in the hydrolysis of ATP to ADP and Pi. - The resulting increase in the concentration of ADP accelerates the rate of reaction that use ADP to generate ATP. - Production of ATP increases until it matches the rate of ATP consumption by energy-requiring reactions.

2 - effect of low ADP: - If ADP (or Pi) is present in limiting concentration, the formation of ATP by oxidative phosphorylation decreases due to lack of phosphate acceptor (ADP) or inorganic phosphate (Pi). - The rate of oxidative phosphorylation is proportinal to [ADP][Pi]/[ATP]. This is known as respiratory control of energy production. - The oxidation of NADH and FADH 2 by the respiratory chain also decline if ADP is limiting. This is because the processes of oxidation and phosphorylation are tightly coupled and must occur at once. - As NADH and FADH 2 accumulate, their oxidized forms become depleted, causing the oxidation of acteyl-Co. A by TCA cycle to be inhibited due to a lack of oxidized coenzymes.

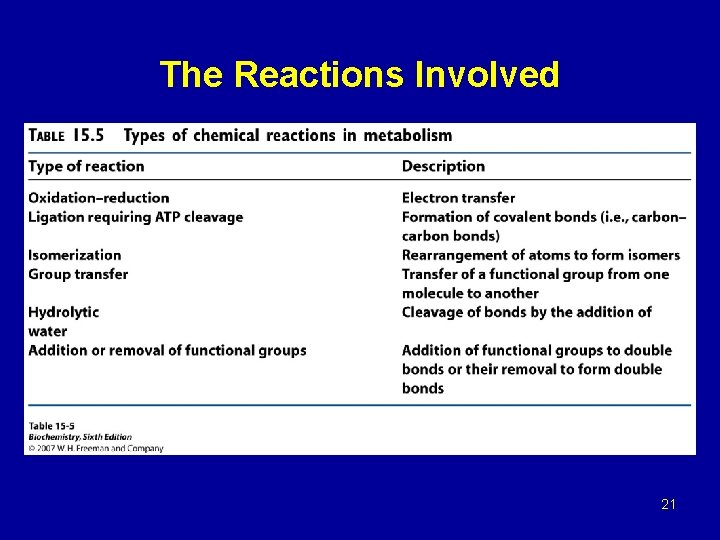
The Reactions Involved 21

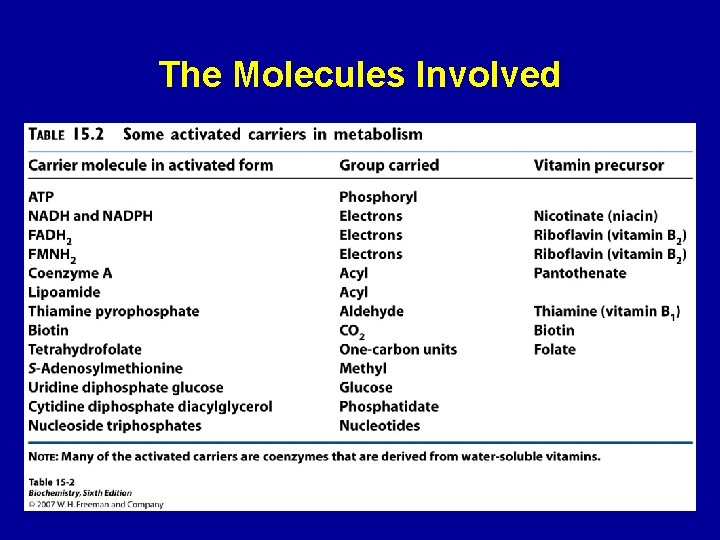
The Molecules Involved 22

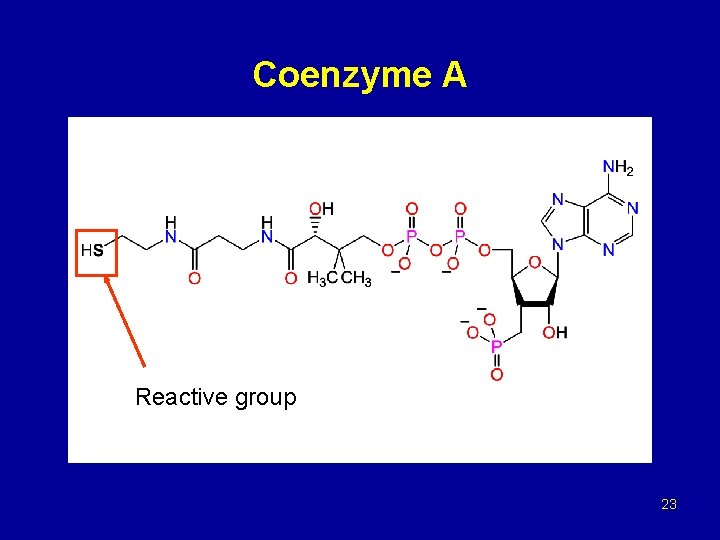
Coenzyme A Reactive group 23

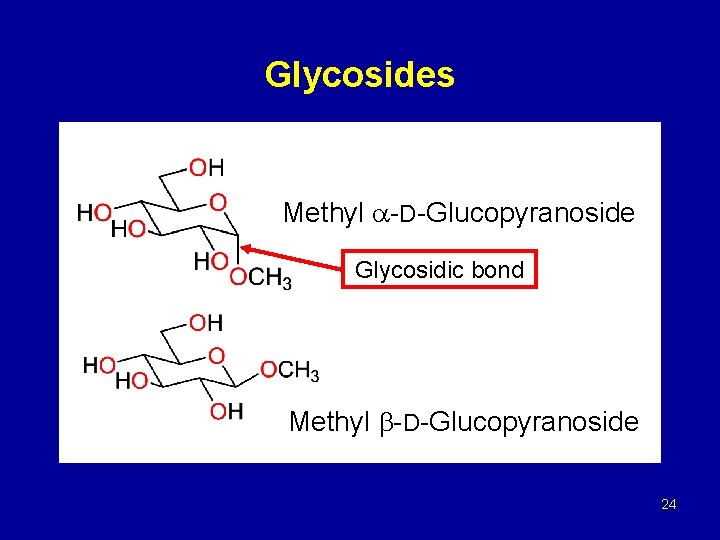
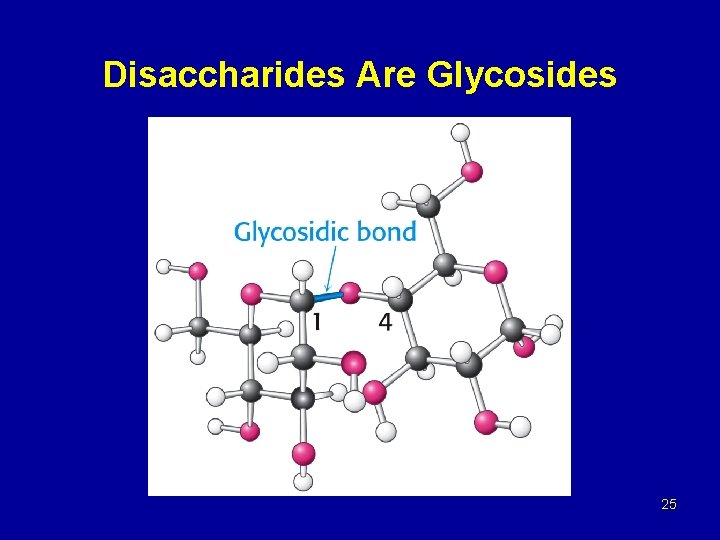
Glycosides Methyl -D-Glucopyranoside Glycosidic bond Methyl -D-Glucopyranoside 24

Disaccharides Are Glycosides 25

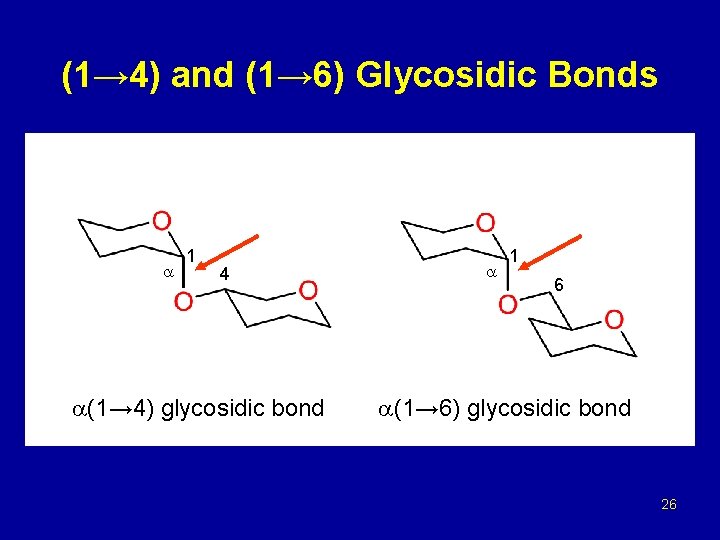
(1→ 4) and (1→ 6) Glycosidic Bonds 1 4 (1→ 4) glycosidic bond 1 6 (1→ 6) glycosidic bond 26

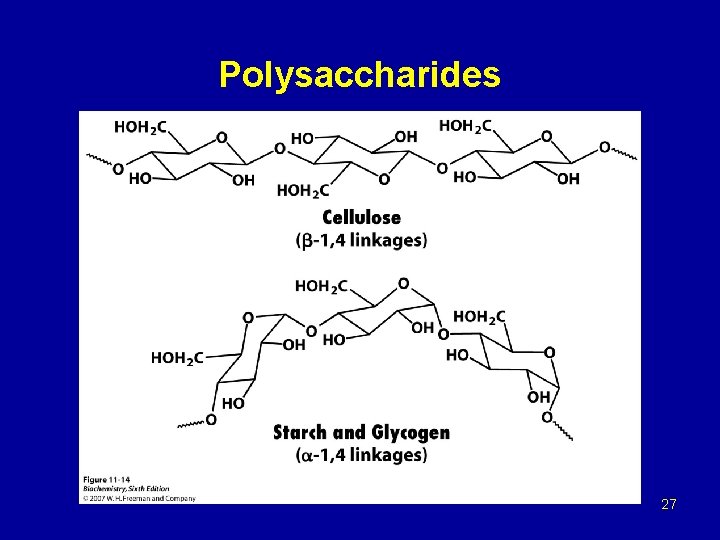
Polysaccharides 27

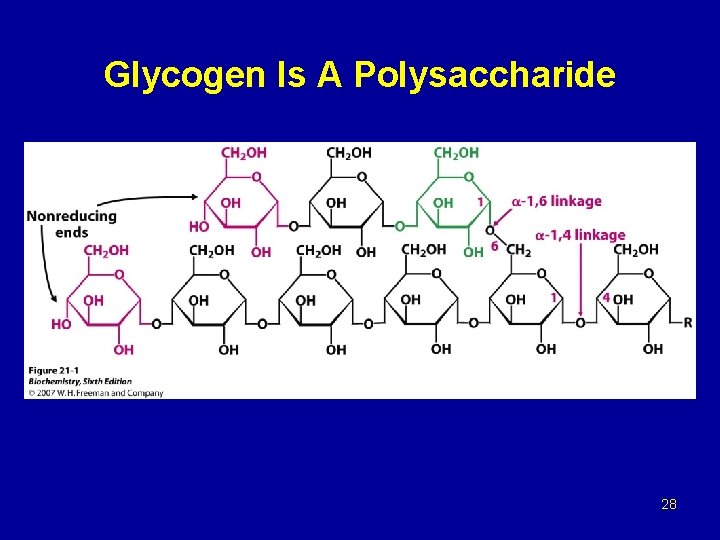
Glycogen Is A Polysaccharide 28

Glycogen metabolism Blood glucose can be obtained from 3 primary sources: - Diet - Degradation of glycogen - Gluconeogenesis In the absence of a dietary source of glucose, glucose is rapidly released from liver glycogen, similarly, muscle glycogen is extensively degraded in working muscle. When glycogen stores are depleted, specific tissues synthesize glucose de novo, using amino acids from the body proteins as the primary source of carbons for the gluconeogenic pathway.

Glycogen breakdown - In muscle, the need for ATP results in the conversion ogf glycogen to glucose 6 -phosphate (G 6 P) for entry into glycolysis. - In liver, low blood glucose concentration triggers glycogen breakdown to G 6 P, which in this case is hydrolyzed to glucose and released into the bloodstream to reverse this situation. - Glycogen breakdown requires the action of three enzymes: 1 - Glycogen phosphorylase catalyzes glycogen phosphorolysis (bond cleavage by the substitution of a phosphate group) to yield glucose 1 -phosphate (G 6 P). Glycogen + Pi (n residues) glycogen + G 1 P (n-1 residues) This enzyme will only release a glucose unit that is at least five units from a branch point.

2 - Glycogen debranching enzyme removes glycogen’s branches, thereby permitting the glycogen phosphotylase reaction to go to completion. It also hydrolyzes alpha(1 -6)-linked glucosyl units to yield glucose. Consequently, ~92% of glycogen’s glucose residues are converted to G 1 P. The remaining ~8%, those at the branch points, are converted to glucose. 3 - Phosphoglucomutae converts G 1 P to G 6 P, which is also formed in the first step of glycolysis through the action of either hexokinase or glucokinase. G 6 P can either continue along the glycolytic pathway (as in muscle) or be hydrolyzed to glucose (as in liver).

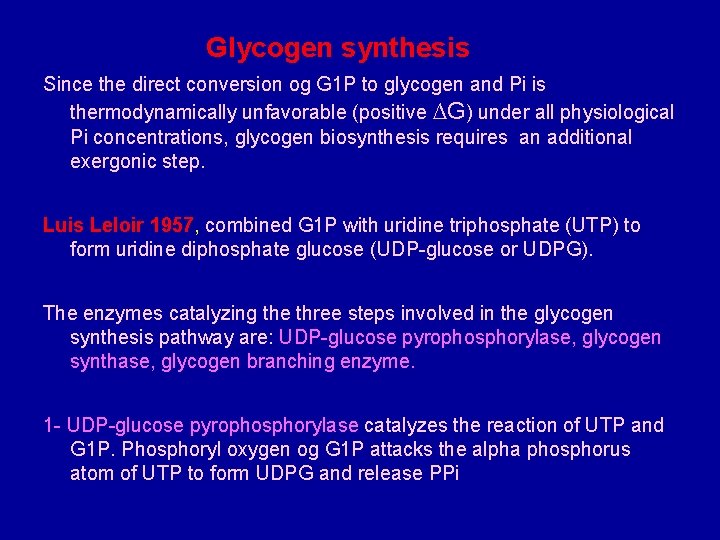
Glycogen synthesis Since the direct conversion og G 1 P to glycogen and Pi is thermodynamically unfavorable (positive ∆G) under all physiological Pi concentrations, glycogen biosynthesis requires an additional exergonic step. Luis Leloir 1957, combined G 1 P with uridine triphosphate (UTP) to form uridine diphosphate glucose (UDP-glucose or UDPG). The enzymes catalyzing the three steps involved in the glycogen synthesis pathway are: UDP-glucose pyrophosphorylase, glycogen synthase, glycogen branching enzyme. 1 - UDP-glucose pyrophosphorylase catalyzes the reaction of UTP and G 1 P. Phosphoryl oxygen og G 1 P attacks the alpha phosphorus atom of UTP to form UDPG and release PPi

2 - Glycogen synthase, the glucosyl unit of UDPG is transferred to the C 4 OH group on one of glycogen’s nonreducing ends to form an alpha(1 -4) glycosidic bond. The glycogen synthase reaction, like those of glycogen phophorylase and lysozyme, is though to involve a glucosyl oxonium ion intermediate or transition state. 3 - Glycogen branching, glycogen synthase catalyzes only α(1 -4) -linkage formation to yield α-amylose. Branching to form glycogen is accomplished by a separate enzyme, amylo-(1, 4 -1, 6)-transglycosylase (branching enzyme), which is distinct from glycogen debranching enzyme. Branches are created by transfer of terminal chain segments consisting of ~7 glucosyl residues to the C 6 -OH groups of glucose residues on the same or another glycogen chain. Each transferred segment must come from a chain of at least 11 residues, and the new branch point must be at least 4 residues away from other branch points.

Summary of glycogen metabolism - In the well-fed state, glycogen synthase is allosterically activated by glucose 6 -phosphate when it is present in elevated concentrations. In contrast, glycogen phophorylase is allosterically inhibited by glucose 6 phosphate, as well as by ATP, a high energy signal in the cell [ Note: in the liver, glucose also serves as an allosteric inhibitor of glycogen phosphorylase]. - Most of the glycogen molecule is degraded to glycose 1 -phosphate by the action of glycogen phosphorylase, the key enzyme in glycogen breakdown. The glycosidic linkage between C-1 of a terminal residue and C-4 of the adjacent one is split by orthophosphate to give glucose 1 -phosphate that can converted into glucose 6 phosphate.

Glycogen is synthesized and degraded by different pathways - Glycogen is synthesized by different pathway from that of glycogen breakdown - UDP-glucose, the activated intermediate in glycogen synthesis, is formed from glucose 1 -phosphate and UDP - Glycogen synthase catalyzes the transfer of glucose from UDP-glucose to the C-4 hydroxyl group of a terminal residue in the growing glycogen molecule - Synthesis is primed by glycogenin, an autoglycosylating protein that contains a covalently attached oligosaccharide unit on a specific tyrosine residue. - A branching enzyme converts some of the alpha-1, 4 linkages into alpha-1, 6 linkages to increase the number of ends so that glycogen can be made and degraded more rapidly.

- In muscle, phosphorylase is activated to generate glucose for use inside the cell as a fuel for contractile activity. - In contrast, liver phosphorylase is activated to liberate glucose for export to other organs, such as skeletal muscle and the brain. - Epinephrine and glucagon stimulate glycogen breakdown through specific 7 TM receptors. - Muscle is the primary target of epinephrine, whereas the liver is responsive to glucagon. Both signal molecules initiate a kinase cascade that lead to the activation of glycogen phosphorylase.

Glycogen breakdown and synthesis are commonly regulated - Glycogen synthesis and degradation are coordinated by several amplifying reaction cascades. - Epinephrine and glucagon stimulate glycogen breakdown and inhibit its synthesis by increasing the cytosolic level of cyclic AMP, which activates protein kinase A. - Elevated cytosolic Ca 2+ levels stimulate glycogen degradation by activating phosphorylase kinase. Therefore, muscle contraction and calcium-mobilizing hormones promote glycogen breakdown. - The glycogen-mobilizing action of PKA are reversed by protein phosphatase 1, that is regulated by several hormones.

- Epinephrine inhibit this phosphatase by blocking its attachment to glycogen molecules and by turning on an inhibitor. - insulin, in contrast, activates this phosphatase by triggering a cascade that phosphorylates that glycogen-targeting subunit of this enzyme. - Hence, glycogen synthesis is decreased by epinephrine and increased by insulin. - Glycogen synthase and phophorylase are also regulated by noncovalent allosteric interactions. - Phosphorylase is a key part of the glucose-sensing system of liver cells. - Glycogen metabolism exemplifies the power and precision of reversible phosphorylation in regulating biological processes.

The metabolism of lipids - Fatty acid β-oxidation – objective - Outline the steps in the β-oxidation and lipogenesis of lipids. - Explain the conditions under which ketone bodies are formed and why - Explain the difference between catabolism and anabolism of lipids - Explain the need for essential fatty acids

Catabolism of lipids – introduction - Fat and glucose are the two major sources of energy for ATP production under most conditions. - Fat provides about half the energy for resting muscle and heart. - The majority of the body’s energy is stored as fat, partly because it is not hydrated and partly because adipose tissue storage appears to be unlimited. - Basically fats converted into acetyl-Co. A and then fed into the citric acid cycle (Krebs).

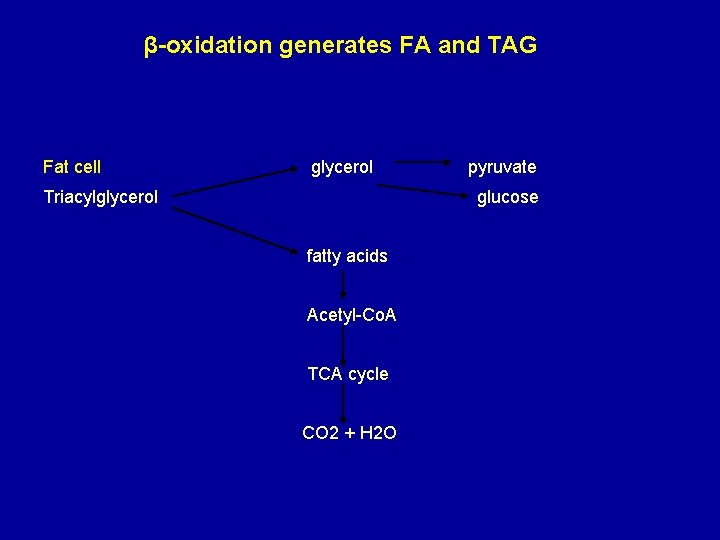
β-oxidation generates FA and TAG Fat cell glycerol Triacylglycerol pyruvate glucose fatty acids Acetyl-Co. A TCA cycle CO 2 + H 2 O

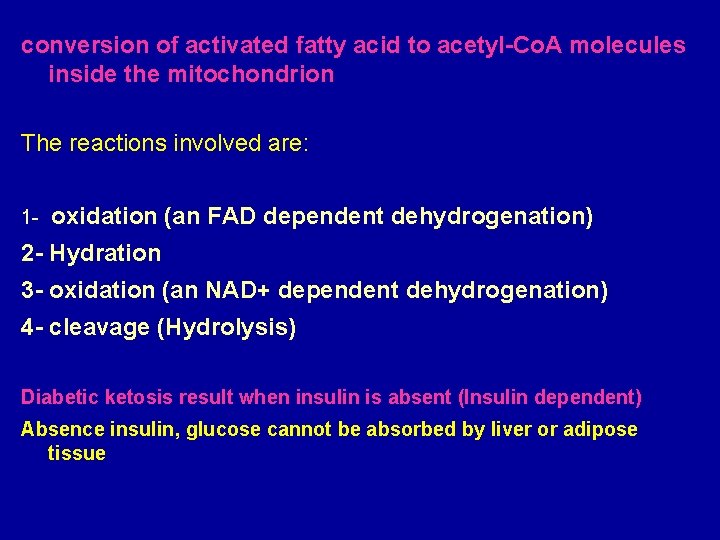
conversion of activated fatty acid to acetyl-Co. A molecules inside the mitochondrion The reactions involved are: 1 - oxidation (an FAD dependent dehydrogenation) 2 - Hydration 3 - oxidation (an NAD+ dependent dehydrogenation) 4 - cleavage (Hydrolysis) Diabetic ketosis result when insulin is absent (Insulin dependent) Absence insulin, glucose cannot be absorbed by liver or adipose tissue

Lipogenesis Anabolism: making lipids Mechanism of fatty acyl-Co. A synthesis 1 - Acetyl-Co. A and malonly-Co. A bond to separate acyl carrier proteins. 2 - Condensation of actyl-ACP and malonyl-ACP to form actoacetyl-ACP. 3 - Reduction by NADPH. 4 - Dehydration. 5 - Reduction by NADPH.

-The cycle now repeats but starting from C 4 rather than C 2, it continues up to C 16. - Aseparate elongation system operates beyond this length. - Steps 2 -4 in lipogenesis are approximate reversals of the corresponding three steps in fatty acid β-oxidation.

Mechanism of fatty acid acyl-Co. A synthesis - The reductant is NADPH rather than NADH - The extra phosphate does not participate in reduction reactions; it is an identification signal, Allows cell to separate reductive power for energy use for synthetic reactions. - NADPH provides reducing power for FA synthesis. - The amount of NADPH generated is only half that which is required for FA synthesis. - The source of the remainder is from the PPP.

Organization of the fatty acid synthesis process - In the bacteria such as E. Coli, there are separate enzymes for each step. - In animals a large enzyme complex called fatty acid synthase carries out all the reactions. The partly synthesized fatty acid remains on the complex until it is complete. - Longer chain fatty acid than palmitate (C 16) are formed by elongation reactions in the endoplasmic reticulum by separate enzymes.

Explain the need for essential fatty acid - Unsaturated fatty acids are required for membrane synthesis and other activities in the body. - Mammals cannot introduce adouble bond beyond C 9 of the FA chain. - Therefore Linoleate (2 double bonds) and Linolenate (3 double bonds) must be included in the diet. - They are essential FA (polyunsaturated) because they are originator of other vital lipids, including prostaglandins.

Summary Fatty acid oxidation - Fatty acid oxidation begins with a dehydrogenation between carbon 2 and 3 of acyl-Co. A derivative. - Next, the trans double bond produced in the first step undergoes enzymatic hydration. - The derivative produced in the second step then undergoes dehydrogenation. There are two final products: 1 - A molecule of acyl-SCo. A from carbons 1 and 2 of the original fatty acid and 2 - Another fatty acyl-SCo. A molecule having 2 fewer carbon atoms than does the original acyl-SCo. A derivative.


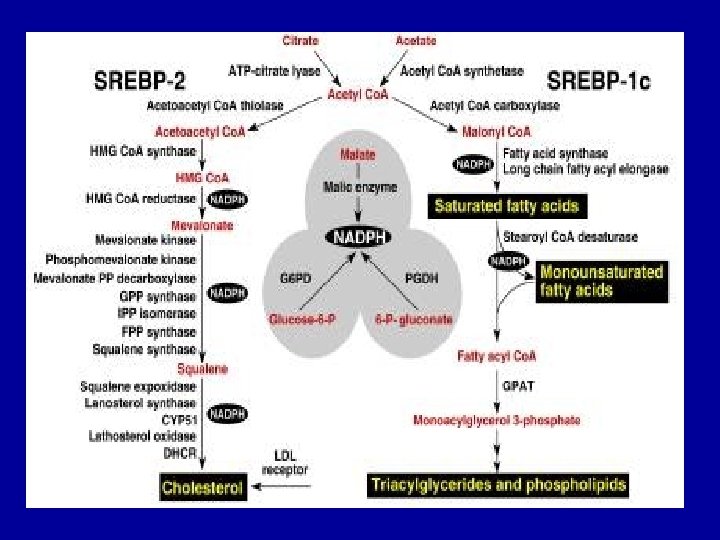
The acetyl-SCo. A formed in this process enters the citric acid cycle and is oxidized to CO 2 and water. The Biosynthesis of fatty acid - The biosynthesis of fatty acids begins when citrate leaves the mitochondria and enters the cytosol where the fatty acid synthase complex lies. - Citrate is both the allosteric signal for the initiation of fatty acid biosynthesis and the origin of acetyl-SCo. A, which is the substrate for the first reaction of malonyl. SCo. A. - The central feature of the fatty acid synthase complex is the presence of two unique-SH groups to which growing fatty acid chains are esterified.

- In the first chain-lengthenine step, acetyl-SCo. A is transferred to the OSH group on the condensing enzyme, and malonyl. SCo. A is transferred to the –SH group on the acetyl carrier protein. - The acetyl group is then condensed with the malonyl group to form the acetoacetyl derivate. At the same time, CO 2 is displace and lost. - The acetyl group becomes the methyl terminal group of the growing chain. - In the next three reactions, the –keto group is reduced, dehydrated, and finally reduced to form an aliphatic chain increased by two carbon atoms.

- The process is repeated until a 16 -carbon chain is formed and released by hydrolysis as palmitoyl-SCo. A. Ketone bodies A significant fraction of the acetyl-Co. A produced by fatty acid oxidation in the liver is converted to acetoacetate and D-beta-hydroxybutyrate, which together with acetone are referred to as ketone bodies. The first two compounds serve as important fuels for the peripheral tissues.

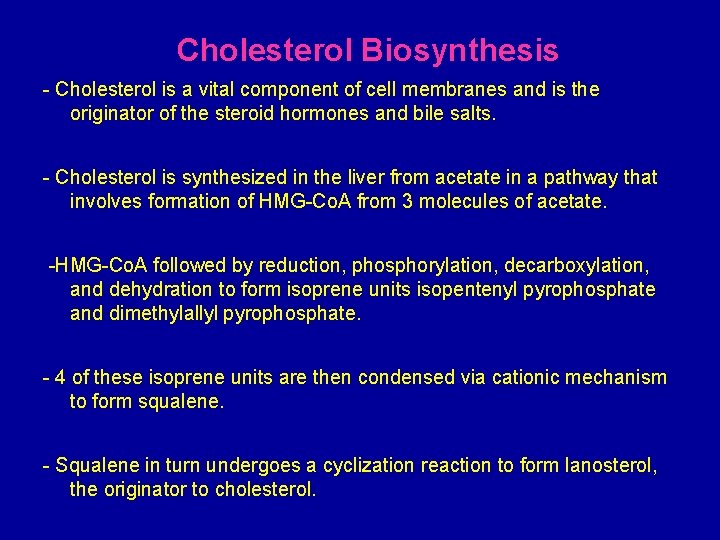
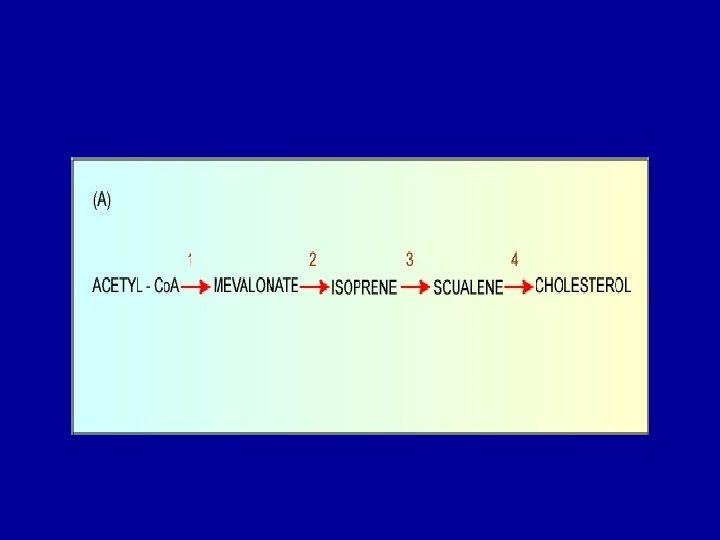
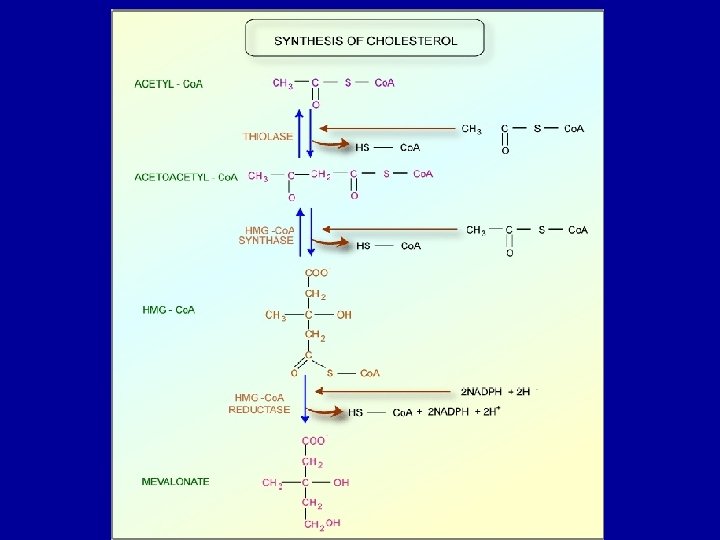
Cholesterol Biosynthesis - Cholesterol is a vital component of cell membranes and is the originator of the steroid hormones and bile salts. - Cholesterol is synthesized in the liver from acetate in a pathway that involves formation of HMG-Co. A from 3 molecules of acetate. -HMG-Co. A followed by reduction, phosphorylation, decarboxylation, and dehydration to form isoprene units isopentenyl pyrophosphate and dimethylallyl pyrophosphate. - 4 of these isoprene units are then condensed via cationic mechanism to form squalene. - Squalene in turn undergoes a cyclization reaction to form lanosterol, the originator to cholesterol.



Biosynthesis and functions of leukotrienes, prostaglandins and phospholipids Leukotrienes, prostaglandins, thromboxanes, and lipoxins are eicosanoid products produced largely by the metabolism of arachidonate. These highly unstable compounds have profound physiological effects at extremely lo concentrations. They are involved in the inflammatory response, the production of pain and fever, the regulation of blood pressure, and many other important physiological processes. Arachidonate is synthesized from linoleic acid, an essential fatty acid, and stored as phosphatidylinositol and other phospholipids.

Prostaglandins and thromboxanes are synthesized via the “cyclic pathway” whereas leukotrienes and lipoxins are synthesized via the “linear pathway. ” Aspirin and other nonsteroidal anti-inflammatory drugs (NSAIDs) inhibit the cyclic pathway but not the linear pathway.