In coordination chemistry the coordination number is the


In coordination chemistry, the coordination number is the number of ligands attached to the central ion (more specifically, the number of donor atoms). Coordination numbers are normally between two and nine. The number of bonds depends on the size, charge, and electron configuration of the metal ion and the ligands. Typically the chemistry of complexes is dominated by interactions between s and p molecular orbitals of the ligands and the d orbitals of the metal ions. The s, p, and d orbitals of the metal can accommodate 18 electrons. The maximum coordination number for a certain metal is thus related to the electronic configuration of the metal ion (specifically, the number of empty orbitals) and to the ratio of the size of the ligands and the metal ion. Large metals and small ligands lead to high coordination numbers (e. g. , [Mo(CN)8]4−). Small metals with large ligands lead to low coordination numbers (e. g. , Pt[P(CMe 3)]2). Due to their large size, lanthanides, actinides, and early transition metals tend to have high coordination numbers. Geometries Different ligand structural arrangements result from the coordination number. Most structures follow the pattern as if the central atom were in the middle and the corners of that shape are the locations of the ligands. These shapes are defined by orbital overlap between ligand metal orbitals and ligand-ligand repulsions, which tend to lead to certain regular geometries. However, there are many cases that deviate from regular geometry. For example, ligands of different sizes and with different electronic effects often result in irregular bond lengths.
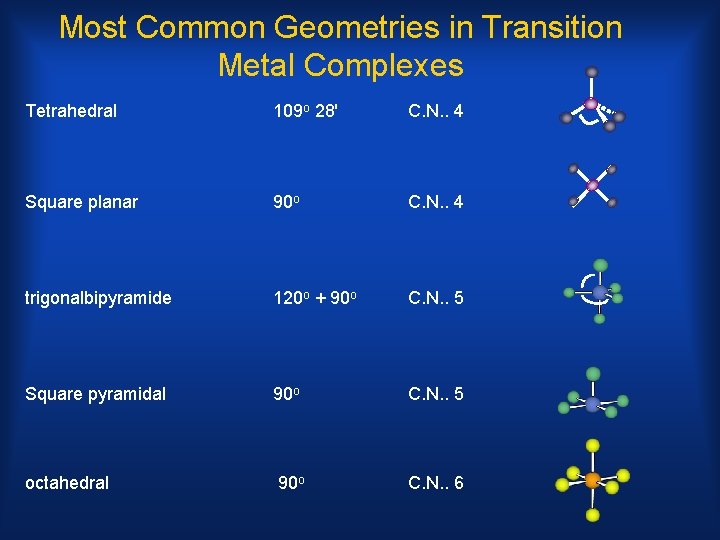
Most Common Geometries in Transition Metal Complexes Tetrahedral 109 o 28' C. N. . 4 Square planar 90 o C. N. . 4 trigonalbipyramide 120 o + 90 o C. N. . 5 Square pyramidal 90 o C. N. . 5 octahedral 90 o C. N. . 6
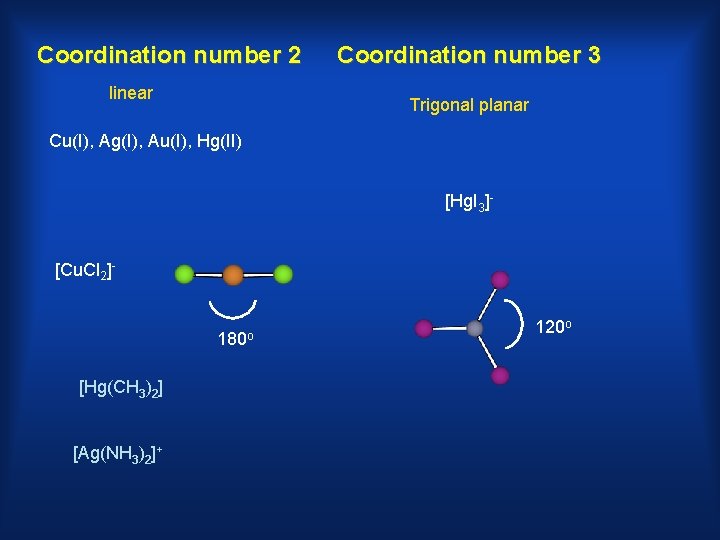
Coordination number 2 linear Coordination number 3 Trigonal planar Cu(I), Ag(I), Au(I), Hg(II) [Hg. I 3]- [Cu. Cl 2]- 180 o [Hg(CH 3)2] [Ag(NH 3)2]+ 120 o
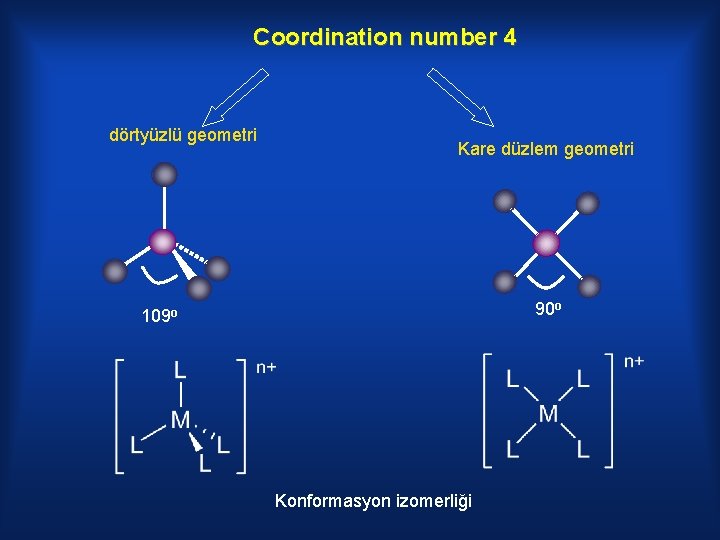
Coordination number 4 dörtyüzlü geometri Kare düzlem geometri 90 o 109 o Konformasyon izomerliği
![Tetrahedral complexes [Co. Cl 4]2[Mn. O 4][Ni. Cl 4]2 - When a cation is Tetrahedral complexes [Co. Cl 4]2[Mn. O 4][Ni. Cl 4]2 - When a cation is](http://slidetodoc.com/presentation_image_h2/0dd800bdc9ea2accdee1e220780bf067/image-6.jpg)
Tetrahedral complexes [Co. Cl 4]2[Mn. O 4][Ni. Cl 4]2 - When a cation is in the following states it gives a tetrehedral complex. 1. When there are ligands with large radii (such as Cl-, Br-, I-) 2 When there are sterically hindered branched ligands 3. When the electron system is in the soildgas system (like Zn 2 +) 4. When the oxidation step of the metal is too high (Cr. O 42 -, Mn. O 4 -))
![Square planar geometry [Pt. Cl 4]2[Au. Br 4][Co(CN)4]2 square-plane complexes occur on d 8 Square planar geometry [Pt. Cl 4]2[Au. Br 4][Co(CN)4]2 square-plane complexes occur on d 8](http://slidetodoc.com/presentation_image_h2/0dd800bdc9ea2accdee1e220780bf067/image-7.jpg)
Square planar geometry [Pt. Cl 4]2[Au. Br 4][Co(CN)4]2 square-plane complexes occur on d 8 metal ions Ni 2+, Pd 2+, Pt 2+, Au 3+ Square plane complexes are formed by strong field ligands CN-

Coordination number 5 Trigonal bipyramid Square pyramid axis position 90 o Equatorial location 120 o The energies of the two structures are very close to each other.
![Trigonal bipyramid Square pyramid 3 - 3 - [Co(CN)5] [Cu. Cl 5]3 - + Trigonal bipyramid Square pyramid 3 - 3 - [Co(CN)5] [Cu. Cl 5]3 - +](http://slidetodoc.com/presentation_image_h2/0dd800bdc9ea2accdee1e220780bf067/image-9.jpg)
Trigonal bipyramid Square pyramid 3 - 3 - [Co(CN)5] [Cu. Cl 5]3 - + [Co(Me 6 tren)Br]+ [VO(acac)2]
![Coordination number 6 Octahedral geometry [Mn(OH 2)6]2+ [Cr(CO)6] The positions of the ligands are Coordination number 6 Octahedral geometry [Mn(OH 2)6]2+ [Cr(CO)6] The positions of the ligands are](http://slidetodoc.com/presentation_image_h2/0dd800bdc9ea2accdee1e220780bf067/image-10.jpg)
Coordination number 6 Octahedral geometry [Mn(OH 2)6]2+ [Cr(CO)6] The positions of the ligands are different Triangular prism geometry WMe 6
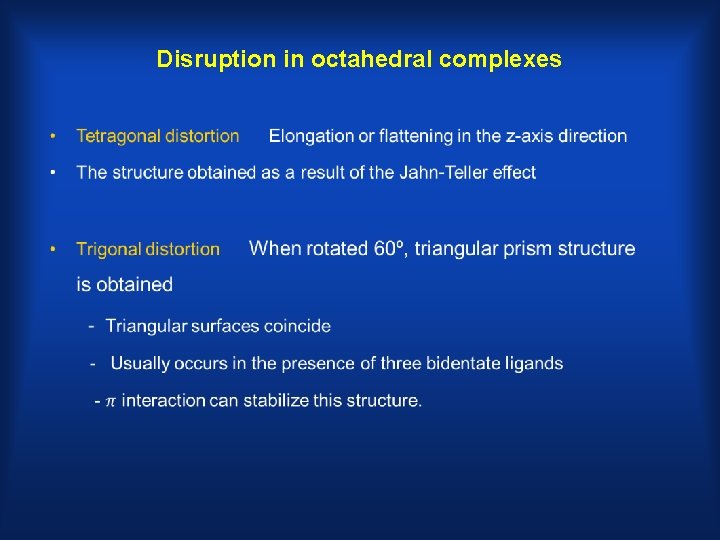
Disruption in octahedral complexes •
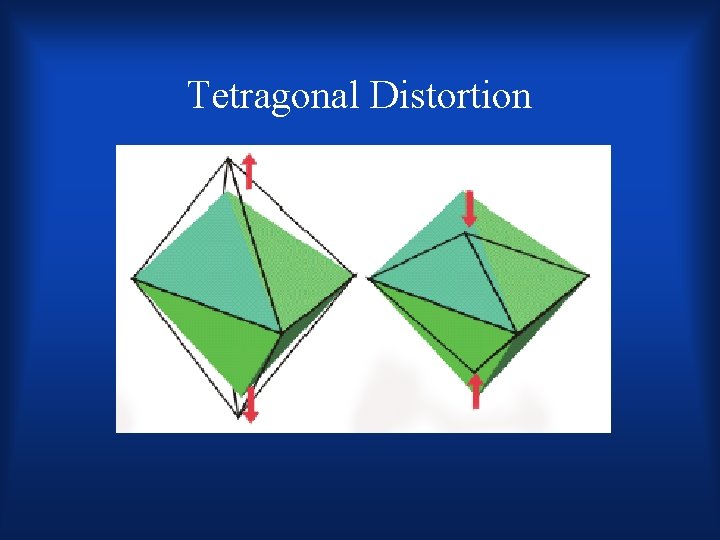
Tetragonal Distortion
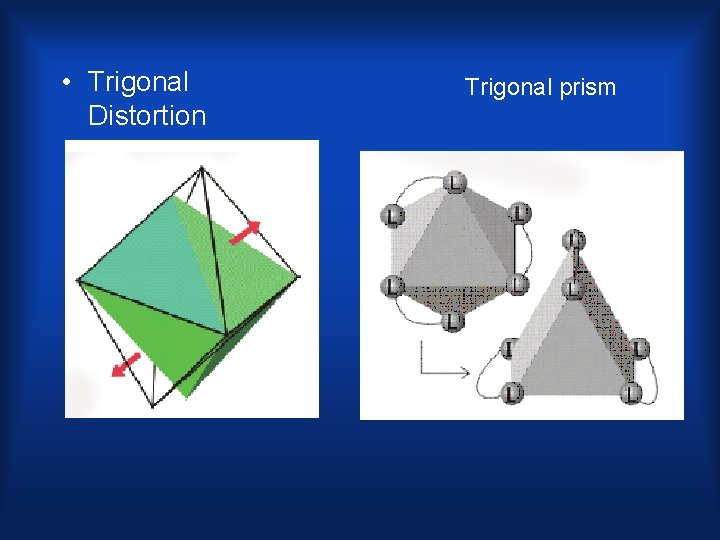
• Trigonal Distortion Trigonal prism
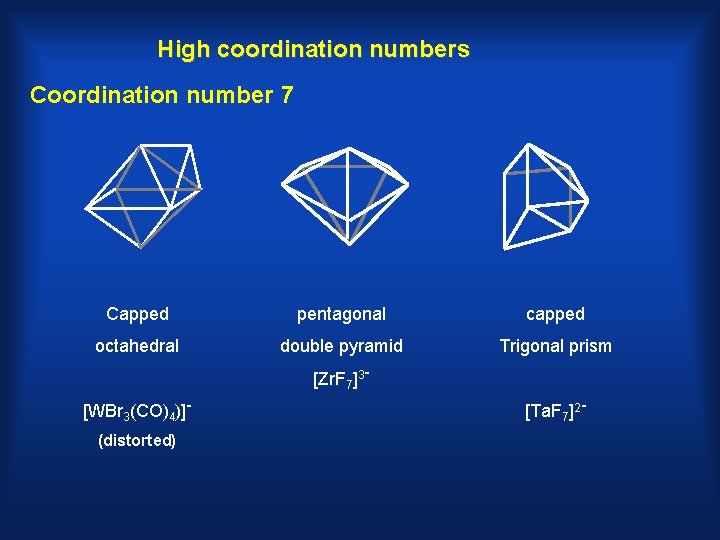
High coordination numbers Coordination number 7 Capped pentagonal capped octahedral double pyramid Trigonal prism [Zr. F 7]3[WBr 3(CO)4)](distorted) [Ta. F 7]2 -
![Coordination number 8 Square antiprism Na 3[Mo(CN)8] Dodecahedron (n. Bu 4 N)3[Mo(CN)8] Coordination number Coordination number 8 Square antiprism Na 3[Mo(CN)8] Dodecahedron (n. Bu 4 N)3[Mo(CN)8] Coordination number](http://slidetodoc.com/presentation_image_h2/0dd800bdc9ea2accdee1e220780bf067/image-15.jpg)
Coordination number 8 Square antiprism Na 3[Mo(CN)8] Dodecahedron (n. Bu 4 N)3[Mo(CN)8] Coordination number 9 Tri-capped trigonal prism [Re. H 9]2 - cube

Coordination numbers and structures Factors determining the structure 1 -Number of bond 2 -VSEPR conditions 3 -Number of electrons in d orbital 4 -Steric barrier of large ligands 5 - Crystal packing effect
- Slides: 16