In association with Translational Research in Oncology Evolving





![Translational Research 2012 clinicaloptions. com/oncology Vemurafenib Inhibition of MAPK Signaling [1] in BRAF V Translational Research 2012 clinicaloptions. com/oncology Vemurafenib Inhibition of MAPK Signaling [1] in BRAF V](https://slidetodoc.com/presentation_image/5df7c65c9bb14bf1646a9418e6a0d509/image-8.jpg)
![Translational Research 2012 clinicaloptions. com/oncology Vemurafenib: Tumor Response by Metastatic Stage Sosman 2012 [2] Translational Research 2012 clinicaloptions. com/oncology Vemurafenib: Tumor Response by Metastatic Stage Sosman 2012 [2]](https://slidetodoc.com/presentation_image/5df7c65c9bb14bf1646a9418e6a0d509/image-10.jpg)
![Translational Research 2012 clinicaloptions. com/oncology Mechanisms of Acquired Resistance to BRAF Inhibitors [1] PDGFRβ Translational Research 2012 clinicaloptions. com/oncology Mechanisms of Acquired Resistance to BRAF Inhibitors [1] PDGFRβ](https://slidetodoc.com/presentation_image/5df7c65c9bb14bf1646a9418e6a0d509/image-17.jpg)








- Slides: 31

In association with Translational Research in Oncology Evolving Treatment Options in Melanoma: Utilizing Genetic Features to Optimize Therapy Antoni Ribas, MD Professor Department of Medicine and Hematology-Oncology University of California, Los Angeles, California This program is supported by educational grants from

Translational Research 2012 clinicaloptions. com/oncology About These Slides § Our thanks to the presenters who gave permission to include their original data § Users are encouraged to use these slides in their own noncommercial presentations, but we ask that content and attribution not be changed. Users are asked to honor this intent § These slides may not be published or posted online without permission from Clinical Care Options Disclaimer The materials published on the Clinical Care Options Web site reflect the views of the authors of the CCO material, not those of Clinical Care Options, LLC, the CME providers, or the companies providing educational grants. The materials may discuss uses and dosages for therapeutic products that have not been approved by the United States Food and Drug Administration. A qualified healthcare professional should be consulted before using any therapeutic product discussed. Readers should verify all information and data before treating patients or using any therapies described in these materials.

Translational Research 2012 clinicaloptions. com/oncology Program Faculty Program Director Faculty Dennis J. Slamon, MD, Ph. D Antoni Ribas, MD TRIO Chairman Chief, Division of Hematology/Oncology David Geffen School of Medicine at UCLA Los Angeles, California Professor Department of Medicine and Hematology-Oncology University of California, Los Angeles, California

Translational Research 2012 clinicaloptions. com/oncology Faculty Disclosures Antoni Ribas, MD, has disclosed that he has received consulting fees from Amgen, Celgene, Genentech/Roche, Glaxo. Smith. Kline, Millennium, Novartis, and Prometheus. Dennis J. Slamon, MD, Ph. D, has disclosed that he has received consulting fees from Genentech, Glaxo. Smith. Kline, and Roche.

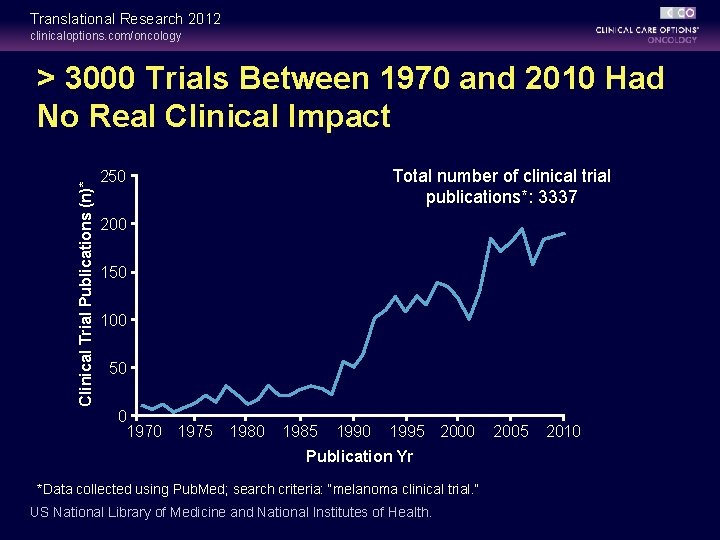
Translational Research 2012 clinicaloptions. com/oncology Clinical Trial Publications (n)* > 3000 Trials Between 1970 and 2010 Had No Real Clinical Impact Total number of clinical trial publications*: 3337 250 200 150 100 50 0 1975 1980 1985 1990 1995 2000 Publication Yr *Data collected using Pub. Med; search criteria: “melanoma clinical trial. ” US National Library of Medicine and National Institutes of Health. 2005 2010

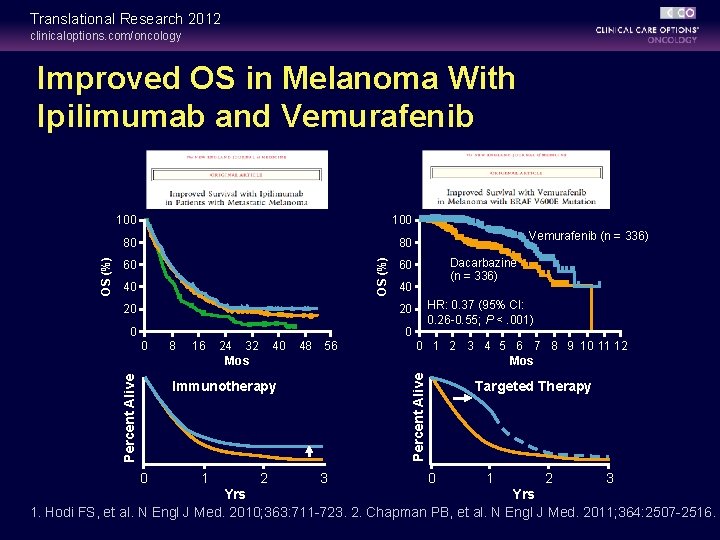
Translational Research 2012 clinicaloptions. com/oncology 100 80 80 OS (%) Improved OS in Melanoma With Ipilimumab and Vemurafenib 60 40 20 Vemurafenib (n = 336) Dacarbazine (n = 336) 60 40 HR: 0. 37 (95% CI: 0. 26 -0. 55; P <. 001) 20 0 0 8 16 24 32 Mos 40 48 56 Immunotherapy 0 1 2 3 4 5 6 7 8 9 10 11 12 Mos Percent Alive 0 3 Targeted Therapy 0 1 2 3 Yrs 1. Hodi FS, et al. N Engl J Med. 2010; 363: 711 -723. 2. Chapman PB, et al. N Engl J Med. 2011; 364: 2507 -2516.

Translational Research 2012 clinicaloptions. com/oncology MAP Kinase Pathway Targeting in NRAS, BRAF mutated in ~ 70% of melanomas, Melanoma c. KIT, usually mutually exclusive [1] < 5% melanomas (mucosal, acral) c. KIT ~ 20% melanomas (> age)[2, 3] NRAS ~ 50% melanomas (< age)[2, 3] BRAF MEK ERK KIT inhibitors: imatinib, nilotinib, dasatinib[4] BRAF inhibitors: vemurafenib, dabrafenib[4] MEK inhibitors: trematenib, TAK 733[4] Oncogenic cell proliferation and survival 1. Sosman JA, et al. ASCO 2011 Educational Book. 2. Arkenau HT, et al. Br J Cancer. 2011; 104: 392 -398. 3. Thomas N, et al. Cancer Epidemiol Biomarkers Prev. 2007; 16: 991 -997. 4. Nikolaou VA, et al. J Invest Dermatol. 2012; 132: 854 -863.
![Translational Research 2012 clinicaloptions comoncology Vemurafenib Inhibition of MAPK Signaling 1 in BRAF V Translational Research 2012 clinicaloptions. com/oncology Vemurafenib Inhibition of MAPK Signaling [1] in BRAF V](https://slidetodoc.com/presentation_image/5df7c65c9bb14bf1646a9418e6a0d509/image-8.jpg)
Translational Research 2012 clinicaloptions. com/oncology Vemurafenib Inhibition of MAPK Signaling [1] in BRAF V 600 Melanoma Biopsies GF Baseline[2] RTK Y-P Ras GTP BRAFV 600 MEK p. ERK Vemurafenib P ERK Cyclin D P Cyclin D Cell cycle (Ki 67) Ki 67 1. Ribas A. Hem. Onc Today: Melanoma. 2012. 2. Flaherty KT, et al. N Engl J Med. 2010; 363: 809 -819. Day 15

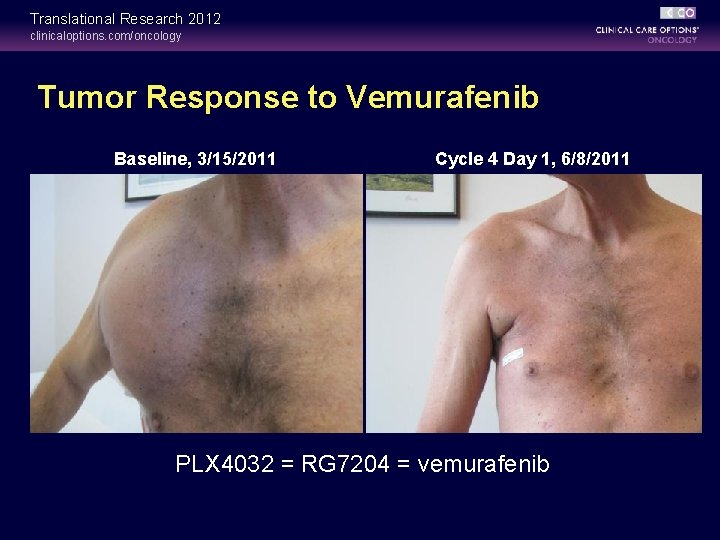
Translational Research 2012 clinicaloptions. com/oncology Tumor Response to Vemurafenib Baseline, 3/15/2011 Cycle 4 Day 1, 6/8/2011 PLX 4032 = RG 7204 = vemurafenib
![Translational Research 2012 clinicaloptions comoncology Vemurafenib Tumor Response by Metastatic Stage Sosman 2012 2 Translational Research 2012 clinicaloptions. com/oncology Vemurafenib: Tumor Response by Metastatic Stage Sosman 2012 [2]](https://slidetodoc.com/presentation_image/5df7c65c9bb14bf1646a9418e6a0d509/image-10.jpg)
Translational Research 2012 clinicaloptions. com/oncology Vemurafenib: Tumor Response by Metastatic Stage Sosman 2012 [2] Flaherty 2010[1] Chapman 2011[3] 1. Flaherty KT, et al. N Engl J Med. 2010; 363: 809 -819. 2. Sosman JA, et al. N Engl J Med. 2012; 366: 707 -714. 3. Chapman PB, et al. N Engl J Med. 2011; 364: 2507 -2516.

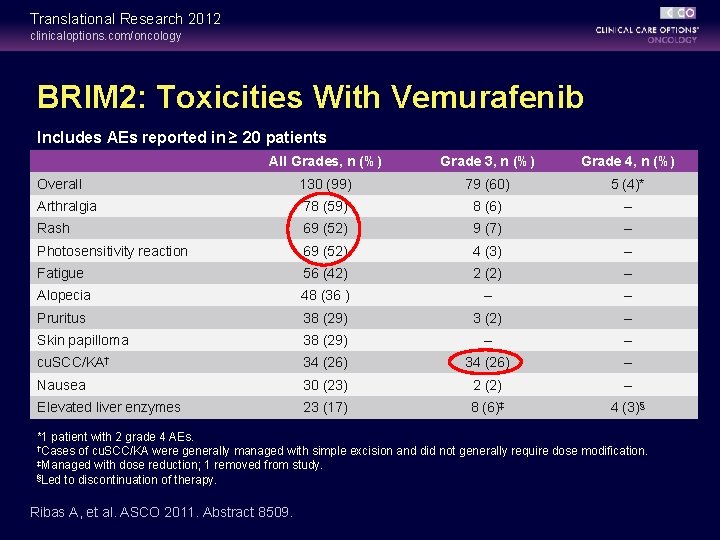
Translational Research 2012 clinicaloptions. com/oncology BRIM 2: Toxicities With Vemurafenib Includes AEs reported in ≥ 20 patients All Grades, n (%) Grade 3, n (%) Grade 4, n (%) Overall 130 (99) 79 (60) 5 (4)* Arthralgia 78 (59) 8 (6) – Rash 69 (52) 9 (7) – Photosensitivity reaction 69 (52) 4 (3) – Fatigue 56 (42) 2 (2) – Alopecia 48 (36 ) – – Pruritus 38 (29) 3 (2) – Skin papilloma 38 (29) – – cu. SCC/KA† 34 (26) – Nausea 30 (23) 2 (2) – Elevated liver enzymes 23 (17) 8 (6)‡ 4 (3)§ *1 patient with 2 grade 4 AEs. †Cases of cu. SCC/KA were generally managed with simple excision and did not generally require dose modification. ‡Managed with dose reduction; 1 removed from study. §Led to discontinuation of therapy. Ribas A, et al. ASCO 2011. Abstract 8509.

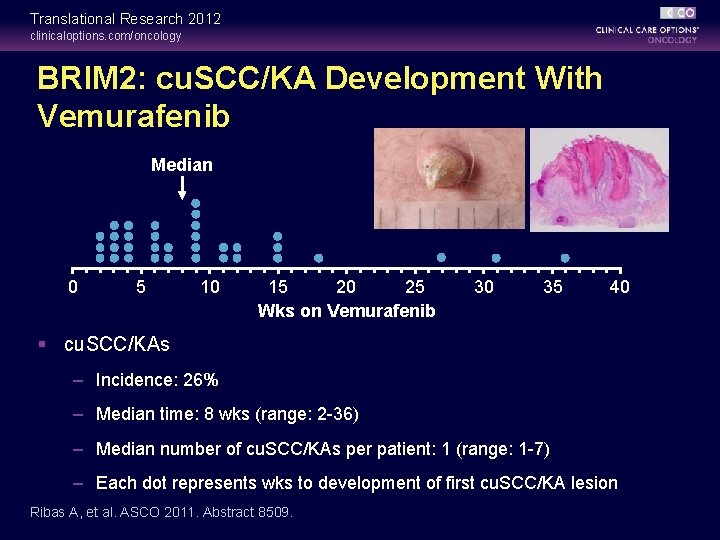
Translational Research 2012 clinicaloptions. com/oncology BRIM 2: cu. SCC/KA Development With Vemurafenib Median 0 5 10 15 20 25 Wks on Vemurafenib 30 35 40 § cu. SCC/KAs – Incidence: 26% – Median time: 8 wks (range: 2 -36) – Median number of cu. SCC/KAs per patient: 1 (range: 1 -7) – Each dot represents wks to development of first cu. SCC/KA lesion Ribas A, et al. ASCO 2011. Abstract 8509.

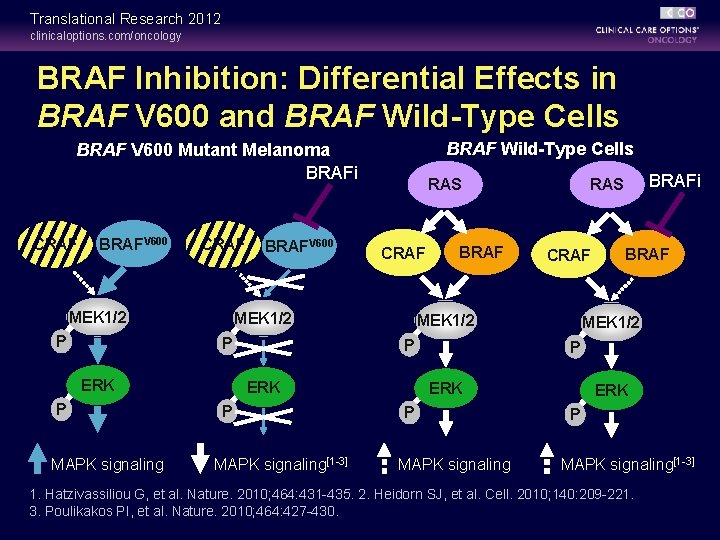
Translational Research 2012 clinicaloptions. com/oncology BRAF Inhibition: Differential Effects in BRAF V 600 and BRAF Wild-Type Cells BRAF V 600 Mutant Melanoma BRAFi CRAF BRAFV 600 CRAF MEK 1/2 P BRAFV 600 ERK MAPK signaling CRAF MEK 1/2 P P RAS BRAF ERK P MAPK signaling[1 -3] CRAF MEK 1/2 P BRAFi RAS BRAF MEK 1/2 P ERK P MAPK signaling[1 -3] 1. Hatzivassiliou G, et al. Nature. 2010; 464: 431 -435. 2. Heidorn SJ, et al. Cell. 2010; 140: 209 -221. 3. Poulikakos PI, et al. Nature. 2010; 464: 427 -430.

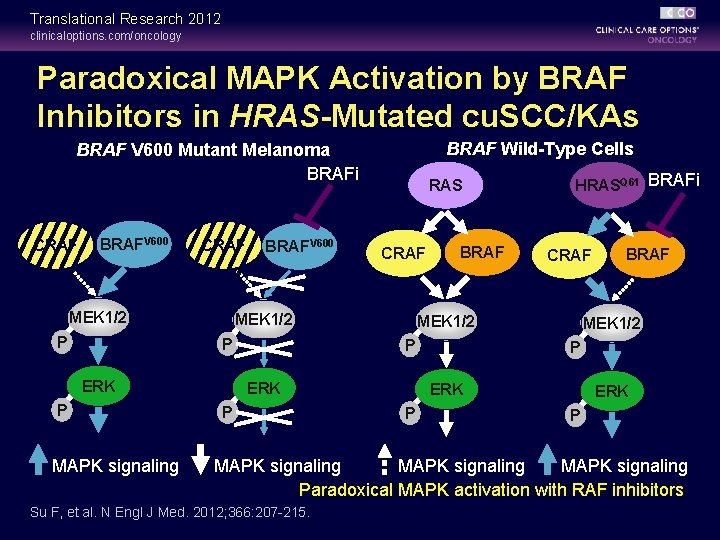
Translational Research 2012 clinicaloptions. com/oncology Paradoxical MAPK Activation by BRAF Inhibitors in HRAS-Mutated cu. SCC/KAs BRAF Wild-Type Cells BRAF V 600 Mutant Melanoma BRAFi CRAF BRAFV 600 CRAF MEK 1/2 P BRAFV 600 ERK MAPK signaling CRAF MEK 1/2 P P RAS BRAF CRAF MEK 1/2 P ERK P HRASQ 61 BRAFi MEK 1/2 P ERK P BRAF ERK P MAPK signaling Paradoxical MAPK activation with RAF inhibitors Su F, et al. N Engl J Med. 2012; 366: 207 -215.

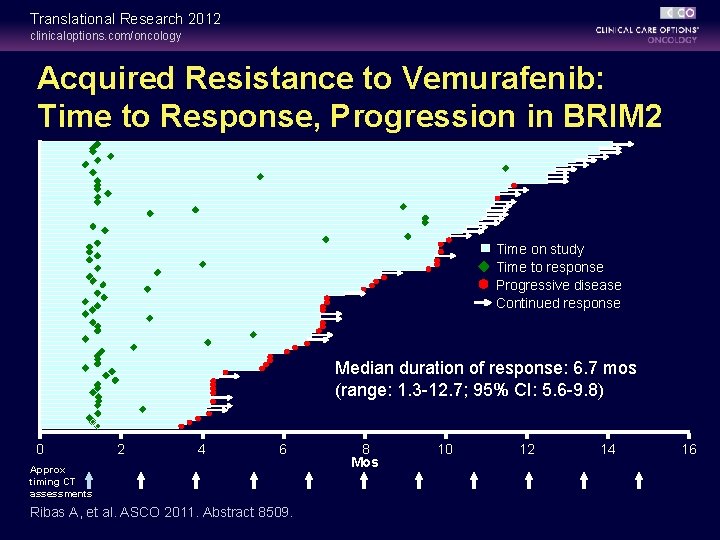
Translational Research 2012 clinicaloptions. com/oncology Acquired Resistance to Vemurafenib: Time to Response, Progression in BRIM 2 Time on study Time to response Progressive disease Continued response Median duration of response: 6. 7 mos (range: 1. 3 -12. 7; 95% CI: 5. 6 -9. 8) 0 2 4 6 Approx timing CT assessments Ribas A, et al. ASCO 2011. Abstract 8509. 8 Mos 10 12 14 16

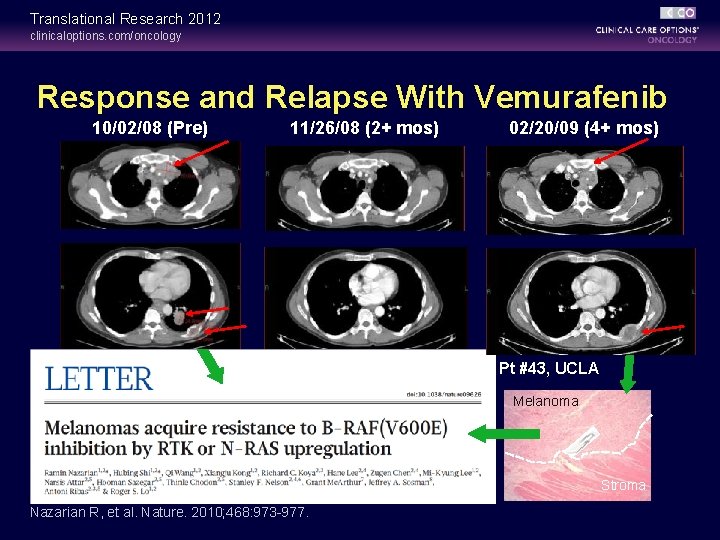
Translational Research 2012 clinicaloptions. com/oncology Response and Relapse With Vemurafenib 10/02/08 (Pre) 11/26/08 (2+ mos) 02/20/09 (4+ mos) Pt #43, UCLA Melanoma Stroma Nazarian R, et al. Nature. 2010; 468: 973 -977.
![Translational Research 2012 clinicaloptions comoncology Mechanisms of Acquired Resistance to BRAF Inhibitors 1 PDGFRβ Translational Research 2012 clinicaloptions. com/oncology Mechanisms of Acquired Resistance to BRAF Inhibitors [1] PDGFRβ](https://slidetodoc.com/presentation_image/5df7c65c9bb14bf1646a9418e6a0d509/image-17.jpg)
Translational Research 2012 clinicaloptions. com/oncology Mechanisms of Acquired Resistance to BRAF Inhibitors [1] PDGFRβ or IGF 1 R or EGFR NRASQ 61 BRAF inhibitor PI 3 K CRAF [2] COT PI 3 Ki or AKTi BRAFV 600 E [3, 4] [4, 6, 9 -11] AKT MEK-dependent progression P MEKi [1, 6 -8] ERK P 1. Nazarian R, et al. Nature 2010. 2. Johannessen CM, et al. Nature. 2010. 3. Poulikakos, et al. Nature 2011. 4. Shi H, et al. Cancer Discov. 2012. 5. Wagle N, et al. J Clin Oncol. 2011. [5] MEK-independent progression Survival 6. Villanueva J, et al. Cancer Cell. 2010. 7. Prahallad A, et al. Nature. 2012. 8. Corcoran RB, et al. Cancer Discov. 2012. 9. Jiang CC, et al. Clin Cancer Res. 2011. 10. Atefi M, et al. PLo. S One. 2011. Su F, et al. Cancer Res. 2012.

Translational Research 2012 clinicaloptions. com/oncology Treating Resistance to BRAFi Occasional prolonged BRAFi Local Tx + BRAFi MEKi BRAFi responses[1] No activity[2] ORR 19%[3] MEKi BRAFi: vemurafenib, dabrafenib MEKi: trametinib ORR 50% to 74%, [4] increased PFS? Progression of melanoma 1. Kim KB, et al. ASCO 2011. Abstract 8519. 2. Kim KB. SMR 2011. Abstract. 3. Flaherty K, et al. SMR 2011. Abstract 12. 4. Infante JR, ASCO 2011. Abstract CRA 8503.

Translational Research 2012 clinicaloptions. com/oncology Metastatic Melanoma: Treatment Advances Immunotherapy Target host Targeted Therapy Target tumor

Translational Research 2012 clinicaloptions. com/oncology Immunotherapy for Melanoma: Response < 5% melanomas, RR 15% KIT inhibitors 50% melanomas, RR 55% BRAF inhibitors < 5% melanomas, RR 30% IL-2 IFN-α Anti-CD 40 Anti-CD 137 Anti-OX 40 MEK inhibitors c. Kit NRAS BRAF MEK ERK Antitumor immune response Anti-CTLA 4 Anti–PD-1 RR ~ 10%, but many are durable Oncogenic cell proliferation and survival

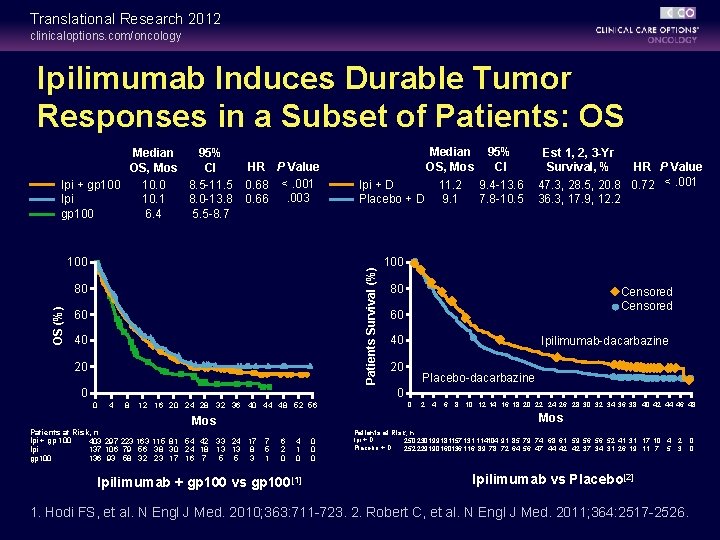
Translational Research 2012 clinicaloptions. com/oncology Ipilimumab Induces Durable Tumor Responses in a Subset of Patients: OS Median 95% OS, Mos CI Ipi + gp 100 10. 0 8. 5 -11. 5 Ipi 10. 1 8. 0 -13. 8 gp 100 6. 4 5. 5 -8. 7 HR P Value 0. 68 <. 001. 003 0. 66 Patients Survival (%) 100 80 OS (%) Median 95% OS, Mos CI 11. 2 9. 4 -13. 6 Ipi + D 7. 8 -10. 5 Placebo + D 9. 1 60 40 20 0 Est 1, 2, 3 -Yr HR P Value Survival, % 47. 3, 28. 5, 20. 8 0. 72 <. 001 36. 3, 17. 9, 12. 2 100 80 Censored 60 40 Ipilimumab-dacarbazine 20 Placebo-dacarbazine 0 0 4 8 12 16 20 24 28 32 36 40 44 48 52 56 2 4 6 8 10 12 14 16 18 20 22 24 26 28 30 32 34 36 38 40 42 44 46 48 Mos Patients at Risk, n Ipi + gp 100 403 297 223 163 115 81 54 42 33 24 17 Ipi 137 106 79 56 38 30 24 18 13 13 8 gp 100 136 93 58 32 23 17 16 7 5 5 3 0 7 5 1 6 2 0 4 1 0 Ipilimumab + gp 100 vs gp 100[1] 0 0 0 Patients at Risk, n Ipi + D 250 230199181157 131 114104 91 85 79 74 68 61 59 56 56 52 41 31 17 10 4 Placebo + D 252 229190160136 116 89 78 72 64 56 47 44 42 42 37 34 31 26 19 11 7 5 2 3 Ipilimumab vs Placebo[2] 1. Hodi FS, et al. N Engl J Med. 2010; 363: 711 -723. 2. Robert C, et al. N Engl J Med. 2011; 364: 2517 -2526. 0 0

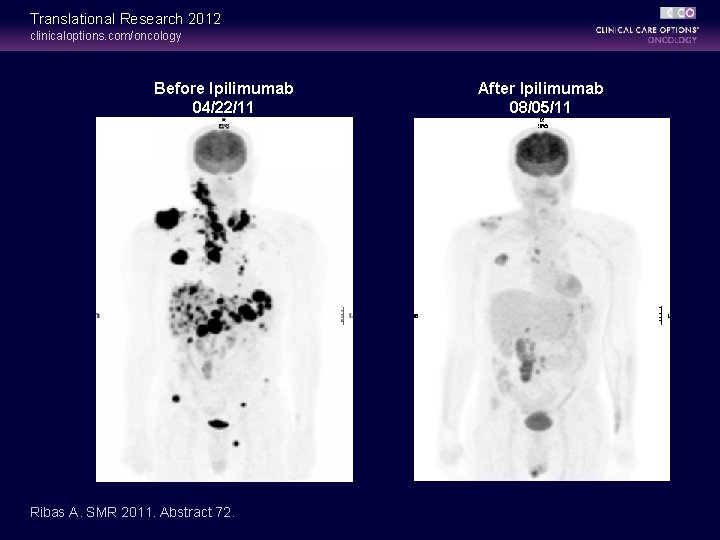
Translational Research 2012 clinicaloptions. com/oncology Before Ipilimumab 04/22/11 Ribas A. SMR 2011. Abstract 72. After Ipilimumab 08/05/11

Translational Research 2012 clinicaloptions. com/oncology “Miracle Survivors”: Reproducible but Low Frequency With CTLA 4 Blocking MAbs CTLA 4 Response Since 2003[1] 1. Forbes. March 2, 2009. 2. Forbes. October 15, 2007. CTLA 4 Response Since 2004[2]

Translational Research 2012 clinicaloptions. com/oncology Anti-PD 1 antibodies may be more relevant to unleash an anti-melanoma immune response than anti-CTLA 4 antibodies Ribas, NEJM June 2, epub ahead of print

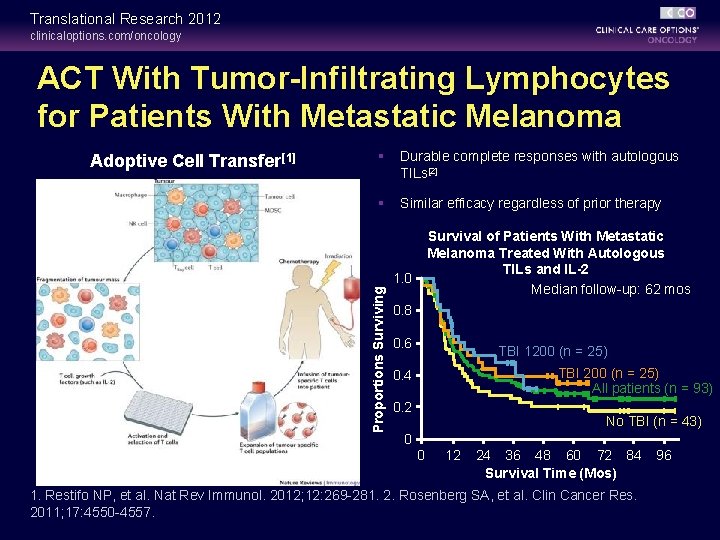
Translational Research 2012 clinicaloptions. com/oncology ACT With Tumor-Infiltrating Lymphocytes for Patients With Metastatic Melanoma Adoptive Cell Transfer[1] § Durable complete responses with autologous TILs[2] § Similar efficacy regardless of prior therapy Survival of Patients With Metastatic Melanoma Treated With Autologous TILs and IL-2 Median follow-up: 62 mos Proportions Surviving 1. 0 0. 8 0. 6 TBI 1200 (n = 25) TBI 200 (n = 25) All patients (n = 93) 0. 4 0. 2 No TBI (n = 43) 0 0 12 24 36 48 60 72 84 Survival Time (Mos) 1. Restifo NP, et al. Nat Rev Immunol. 2012; 12: 269 -281. 2. Rosenberg SA, et al. Clin Cancer Res. 2011; 17: 4550 -4557. 96

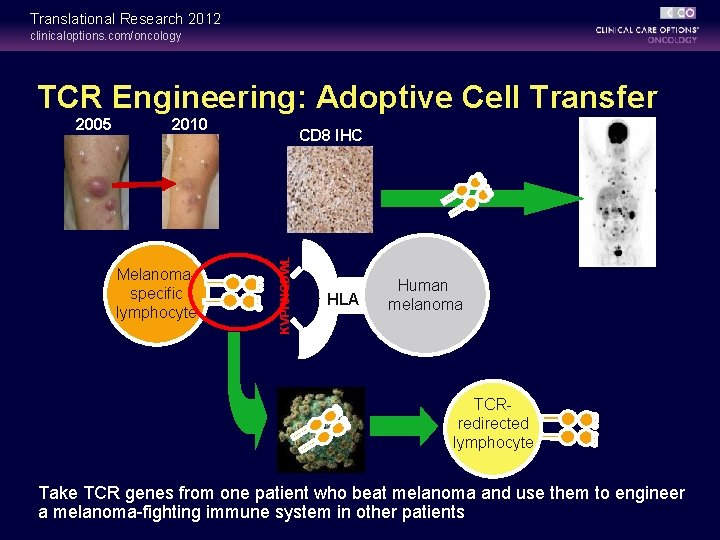
Translational Research 2012 clinicaloptions. com/oncology TCR Engineering: Adoptive Cell Transfer 2010 Melanomaspecific lymphocyte CD 8 IHC KVPRNQDWL 2005 HLA Human melanoma TCRredirected lymphocyte Take TCR genes from one patient who beat melanoma and use them to engineer a melanoma-fighting immune system in other patients

Translational Research 2012 clinicaloptions. com/oncology Immunotherapy for Melanoma Active immunotherapy § Cytokines Adoptive cell transfer § T-cell cloning – IL-2 § – From blood – From tumors: TIL Antibodies § – Anti-CTLA 4 T-cell genetic engineering – T-cell receptors – Chimeric antigen receptors General clinical effects § Low frequency of tumor responses § Highly durable § IL-2: limited by short-term toxicities § Anti-CTLA 4: limited by midterm autoimmune toxicities § High frequency of tumor responses § Variable durability § Limited by requirements of preparative conditioning and IL-2

Translational Research 2012 clinicaloptions. com/oncology 100 80 80 OS (%) Combining Immunotherapy and Targeted Therapy for Melanoma? 60 40 20 40 0 0 8 16 24 32 Mos 40 56 HR: 0. 37 (95% CI: 0. 26 -0. 55; P < 0. 001) 0 1 2 3 4 5 6 7 8 9 10 11 12 Mos Combination Targeted Therapy 0 1 2 3 Percent Alive Immunotherapy 48 Dacarbazine (n = 336) 60 20 0 Vemurafenib (n = 336) 0 1 2 3 ? 0 1 2 3 Yrs Yrs 1. Hodi FS, et al. N Engl J Med. 2010; 363: 711 -723. 2. Chapman PB, et al. N Engl J Med. 2011; 364: 2507 -2516.

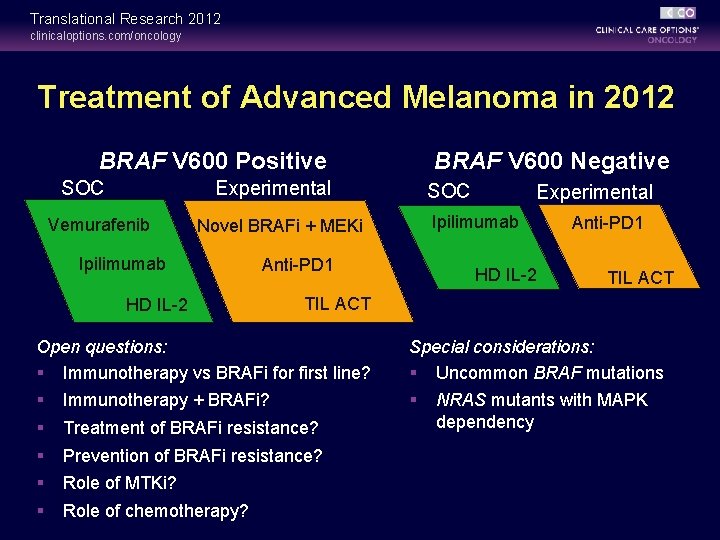
Translational Research 2012 clinicaloptions. com/oncology Treatment of Advanced Melanoma in 2012 BRAF V 600 Positive BRAF V 600 Negative Experimental SOC Vemurafenib SOC Ipilimumab Novel BRAFi + MEKi Ipilimumab Anti-PD 1 HD IL-2 Experimental HD IL-2 Anti-PD 1 TIL ACT Open questions: § Immunotherapy vs BRAFi for first line? Special considerations: § Uncommon BRAF mutations § § § Immunotherapy + BRAFi? Treatment of BRAFi resistance? Prevention of BRAFi resistance? Role of MTKi? Role of chemotherapy? NRAS mutants with MAPK dependency

Translational Research 2012 clinicaloptions. com/oncology Conclusions § “ 2011: The year of melanoma” – George Sledge, MD, President of ASCO § Scientific advances translate into improved patient care § The mechanism of resistance to BRAF inhibitors predicts sensitivity to the addition of secondary treatments – MEK inhibitors – PI 3 K/AKT/m. TOR inhibitors § Combining immunotherapy and BRAF-targeted therapy in the clinic is warranted

Go Online for More CCO Coverage of Chicago 2012! Capsule Summaries of all the key data, plus CME-certified Slidesets exploring the clinical implications of these findings Downloadable slides: for use as a study resource or in your noncommericial presentations clinicaloptions. com/oncology
 Primary control vs secondary control
Primary control vs secondary control Evolving design
Evolving design A framework for clustering evolving data streams
A framework for clustering evolving data streams Key evolving signature
Key evolving signature Evolving
Evolving A body is in translational equilibrium if
A body is in translational equilibrium if Lj wei
Lj wei Translational kinetic energy
Translational kinetic energy Translational vs rotational motion
Translational vs rotational motion Post translational and co translation
Post translational and co translation Oscillatory motion example
Oscillatory motion example Translational kinetic energy
Translational kinetic energy Translational kinetic energy formula
Translational kinetic energy formula Translational motion definition
Translational motion definition Translational kinetic energy
Translational kinetic energy Translational vs rotational motion
Translational vs rotational motion Translational criminology
Translational criminology What is translational motion
What is translational motion Translational speed formula
Translational speed formula Answer
Answer Duke translational medicine institute
Duke translational medicine institute Rotational motion
Rotational motion Translational slide
Translational slide Ap physics unit 7 mcq part a
Ap physics unit 7 mcq part a Translational motion equations
Translational motion equations Translational force
Translational force Partition formula geometry
Partition formula geometry Translational motion diagram
Translational motion diagram The first condition of equilibrium implies that: phy101
The first condition of equilibrium implies that: phy101 Translational mechanical system transfer functions
Translational mechanical system transfer functions Katharina reiss functional theory
Katharina reiss functional theory Translational fx
Translational fx