In an ionic compound positive charges must balance

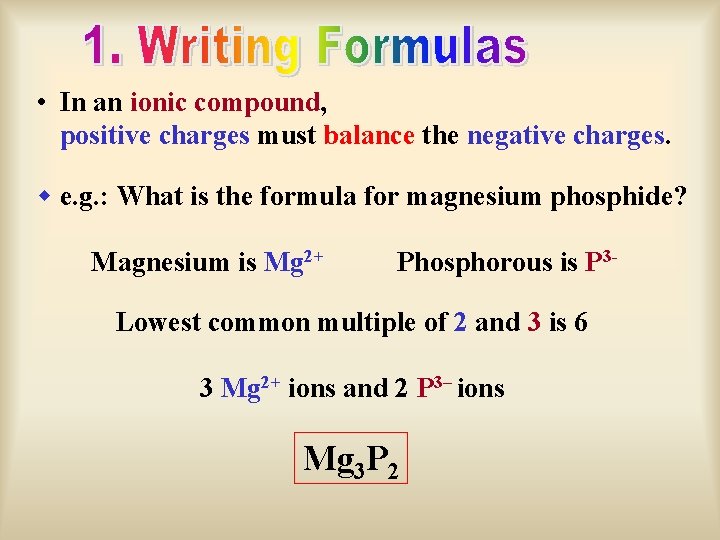
• In an ionic compound, positive charges must balance the negative charges. w e. g. : What is the formula for magnesium phosphide? Magnesium is Mg 2+ Phosphorous is P 3 - Lowest common multiple of 2 and 3 is 6 3 Mg 2+ ions and 2 P 3– ions Mg 3 P 2
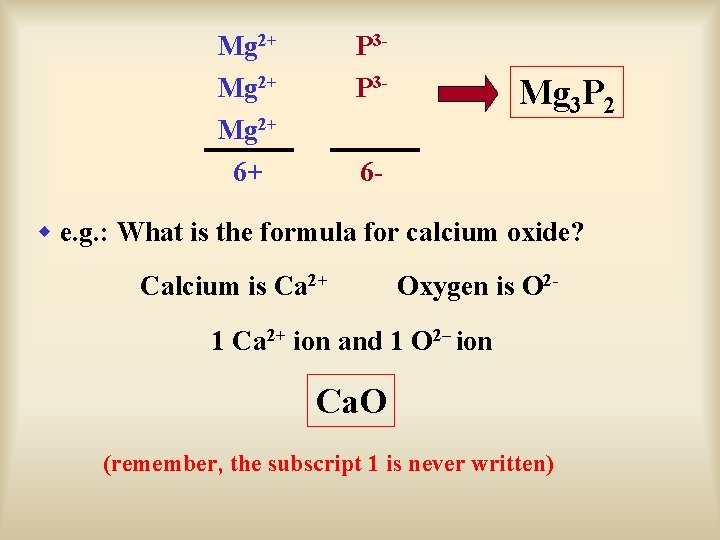
Mg 2+ P 3 P 3 - 6+ 6 - Mg 3 P 2 w e. g. : What is the formula for calcium oxide? Calcium is Ca 2+ Oxygen is O 2 - 1 Ca 2+ ion and 1 O 2– ion Ca. O (remember, the subscript 1 is never written)
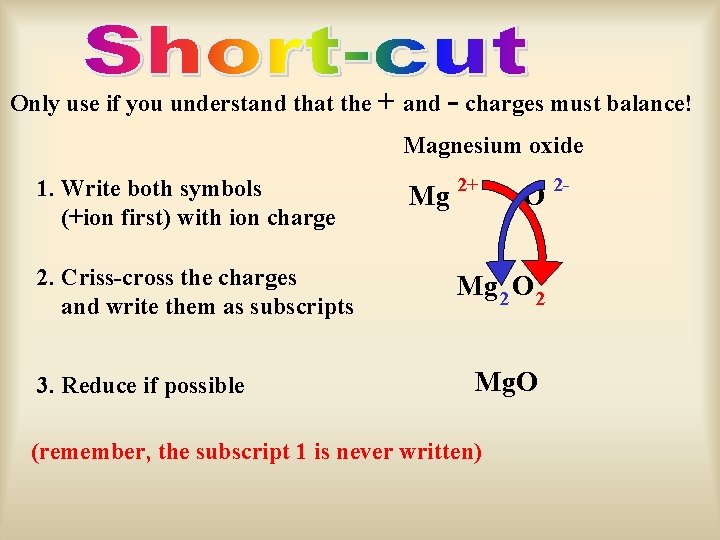
Only use if you understand that the + and - charges must balance! Magnesium oxide 1. Write both symbols (+ion first) with ion charge 2. Criss-cross the charges and write them as subscripts 3. Reduce if possible Mg 2+ O Mg 2 O 2 Mg. O (remember, the subscript 1 is never written) 2 -
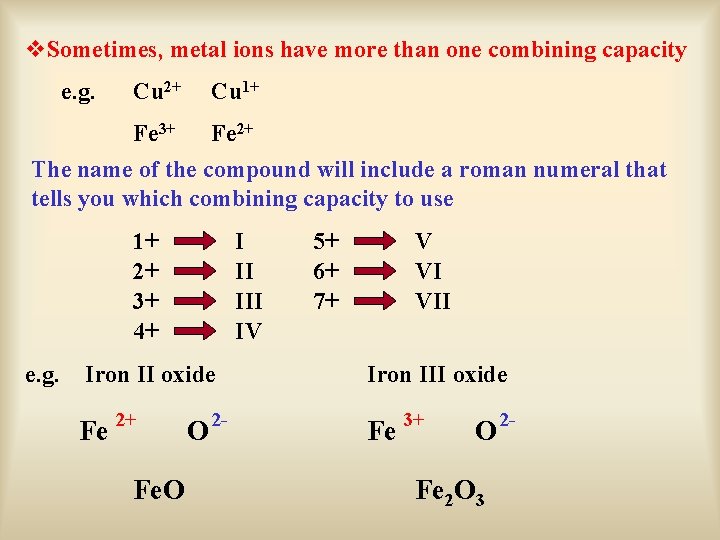
v. Sometimes, metal ions have more than one combining capacity e. g. Cu 2+ Cu 1+ Fe 3+ Fe 2+ The name of the compound will include a roman numeral that tells you which combining capacity to use 1+ 2+ 3+ 4+ e. g. I II IV 5+ 6+ 7+ V VI VII Iron II oxide Iron III oxide Fe 2+ Fe 3+ Fe. O O 2 - Fe 2 O 3

1. Write the name of the metal first. 2. Write the name of the non-metal second, and change the ending to “-ide” 3. ONLY IF THE METAL HAS 2 OR MORE ION CHARGES, Include a roman numeral to indicate which one it is. e. g. Na. Cl Sodium chloride Mg. F 2 Magnesium fluoride Cu. Cl 2 copper has 2 ions: which one is it? Cu 1+ 2+ Cu
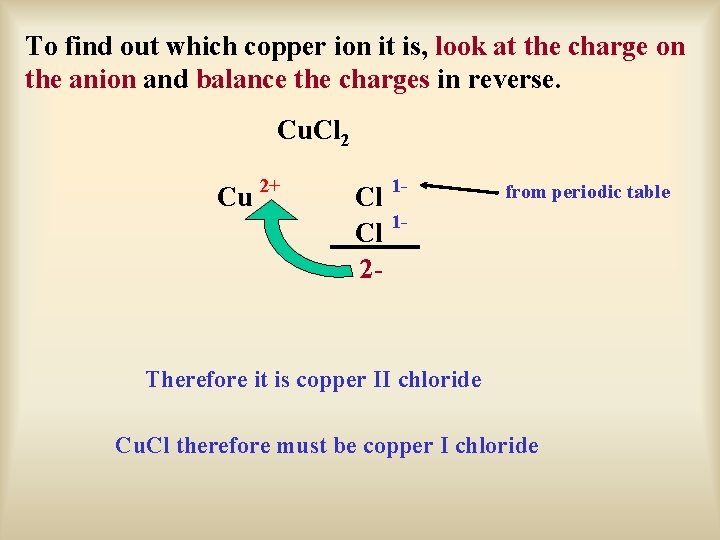
To find out which copper ion it is, look at the charge on the anion and balance the charges in reverse. Cu. Cl 2 Cu 2+ Cl 12 - from periodic table Therefore it is copper II chloride Cu. Cl therefore must be copper I chloride
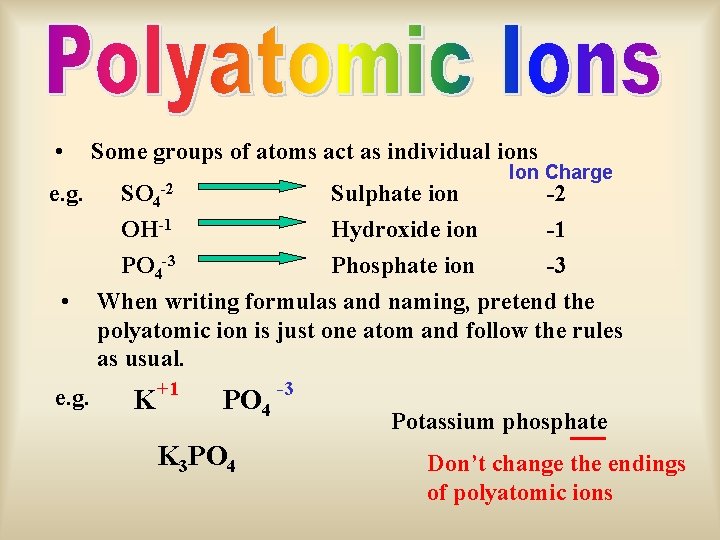
• Some groups of atoms act as individual ions e. g. SO 4 -2 Sulphate ion -2 OH-1 Hydroxide ion -1 PO 4 -3 Phosphate ion -3 When writing formulas and naming, pretend the polyatomic ion is just one atom and follow the rules as usual. • e. g. Ion Charge K +1 PO 4 K 3 PO 4 -3 Potassium phosphate Don’t change the endings of polyatomic ions

Al +3 SO 4 -2 Al 2(SO 4)3 Aluminum sulphate Put brackets around the SO 4 to indicate 3 sulphate ions, not 43 oxygen atoms Cu +2 SO 4 -2 Cu. SO 4 Copper (II) sulphate Don’t forget the roman numeral since copper has more than one combining capacity
- Slides: 9