Improving the renewal process for active substances already

Improving the renewal process for active substances already known to meet the exclusion criteria CA-Feb 20 -Doc. 5. 3 1

Sum up of the last Standing Committees’ discussions • Questions discussed : • • Can the evaluation of the conditions for derogation to exclusion of Article 5(2) be made before any extension is granted? Can the evaluation of the conditions for derogation to exclusion of Article 5(2) to exclusion be made before requesting any additional data on EDs? Is the evaluation of ED properties necessary if the AS already meet other exclusion criteria? How can the process be adapted to complete the examination of the renewal in the most efficient manner? • It is clear that the Commission has the legal obligation to extend the approval of an active substance if examination of the renewal is not finalised before the expiry date of approval • General agreement that we need to find ways to speed up the process for these substances, and speed up the analysis of alternatives • The legal framework of the BPR has to be respected 2

Reflexions on ways to speed up the renewal process • Actions already agreed : • CA-July 17 -Doc. 5. 3 - Final - AS renewals 2016 -2020. docx Ø Pre-submission meetings, early discussions between the e. CA and the prospective applicant (data package, reason of submission and derogation to exclusion, ED data etc. . ) Ø Burden on proof on the necessity of the AS on the applicant: it needs to provide in its application information and detailed justifications on the necessity of the substance, for which specific use(s) within the PT, and its justifications as for why one or more criteria for derogation to exclusion of Article 5(2) would be met this(/these) use(s) • Legal obligation : the e. CA has to decide within 90 days whether it intends to perform a limited or a full evaluation it shall inform COM and ECHA of its decision • When an approval is likely to expire before completion of the renewal process: an extension will now be granted to one year for AS meeting exclusion criteria, renewable if necessary (MS need to extend, and renew extensions, for their BP authorisations as necessary). This approach may be revised in the light of experience. 3

Reflexions on ways to speed up the renewal process • Possible actions for further discussion (not yet agreed): • After the acceptance of the application by ECHA: ü ECHA could start its public consultation on the availability of alternatives (similar to consultations under Article 10(3) for first approval) ü The Commission could request a specific opinion of ECHA under Article 75(1)(g) concerning the availabilities of alternatives, to be submitted within 3 – 6 months? • In parallel to the scientific assessment by the e. CA, discussions on the possibilities for derogation to exclusion could start: ü The e. CA shall start its own assessment of the conditions of Article 5(2) ü Other Member States should also look at this information in the application ü A discussion could be organised at Standing Committee level to get the views of other Member States (without prior public consultation) ü 3 months after the acceptance of the application by ECHA? ü Or after the reception of the ECHA opinion on the availability of alternatives? (if requested) This could give preliminary indications to the e. CA on whether its assessment could be finalised without requesting further information, and speed up its work. 4

Reflexions on ways to speed up the renewal process • Possible actions for further discussion (not yet agreed): • Issues of discussions on the availability of alternatives, and derogation conditions under Article 5(2), so far: ü Variable input from applicants on Article 5(2) justifications ü Limited contributions received from stakeholders during public consultations, even from competitors ü Limited contributions during ECHA BPC discussions and public consultations from Member States ü Limited input from ECHA BPC opinion on alternatives during the first approvals processes despite former agreements (CA-May 16 -Doc. 5. 3 - Final - Public consultation & BPC input. doc) ü Knowing if an AS has ED properties may influence the assessment of the conditions for derogation under Article 5(2) (a) and (c), and possible conditions for renewal. ü Priority lists in the review programme are not sufficiently respected, which creates additional difficulties in comparing active substances when it comes to renewals Need for improvements, possible ways : Better respect of priority lists? Better anticipation? MS to better communicate to their stakeholders? Stakeholders associations to better contribute, and communicate to their affiliates? MS to analyse their own markets and share the information at EU level (during public consultation, BPC, SCBP)? ü Studies on alternatives, workshops to be performed on PTs : COM? ECHA? MS? Stakeholder associations? Workload shared across EU/actors? ü ü ü 5

Reflexions on ways to speed up the renewal process • Possible actions for further discussion (not yet agreed): • Main technical cause of possible delays in the renewal of approval : ED assessment • Fact : ED data are Core Data Set in the BPR, and the applicant has the obligation to provide sufficient information in its application in order to make it possible to assess ED properties • What if submitted data does not allow to reach a conclusion on ED properties? 6

Reflexions on ways to speed up the renewal process • Possible actions for further discussion (not yet agreed): v When a non-renewal of approval is proposed due to other matters (unaccepatble risks, no derogation of Article 5(2) possible), no need to request further assess ED properties which would only delay the process. v On the contrary, when an approval may be possible and the submitted and available data does not allow to draw a conclusion on ED properties, on-going internal discussions on whether additional data have to be requested in any case to reach a conclusion. Legal considerations Opportunity considerations: advantages/disadvantages of having a definitive conclusion on ED compared to advantages/disadvantages of taking already a decision on a renewal? In case it could be possible to not request further ED data, would MS accept adopting decisions without definitive conclusions on ED properties? 7

Conclusion 8

Reminders : provisions of BPR • • • Submission legal deadline : 1, 5 years before the expiry Basis for the renewal decision: BPC opinion, following the submission of the draft Assessment Report from the e. CA The e. CA must decide on the basis of available information within 3 months after the acceptance of the application by ECHA whether it intends to perform a full or limited evaluation The e. CA must give, in its draft assessment, a recommendation on the renewal of approval of the active substance, which therefore includes a draft evaluation of the conditions for derogation to exclusion of Article 5(2) The conditions for derogation of Article 5(2)(c) includes an assessment whether the ban would have disproportionate negative impact on society when compared to the risks of using the substance In the assessment of the conditions of Article 5(2), the availability of suitable and sufficient alternatives is a key consideration 9
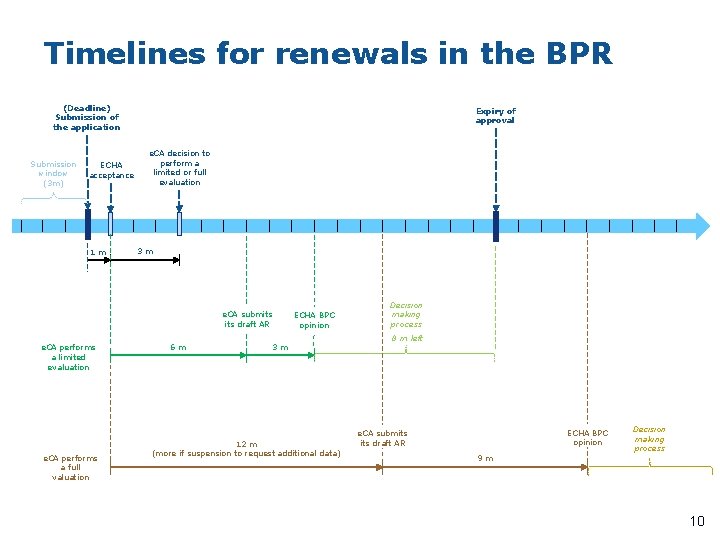
Timelines for renewals in the BPR (Deadline) Submission of the application Submission window (3 m) ECHA acceptance 1 m Expiry of approval e. CA decision to perform a limited or full evaluation 3 m e. CA submits draft AR e. CA performs a limited evaluation e. CA performs a full valuation 6 m ECHA BPC opinion 3 m 12 m (more if suspension to request additional data) Decision making process 8 m left ECHA BPC opinion e. CA submits draft AR Decision making process 9 m 10
- Slides: 10