Improving Care and Collaboration for Children with Neurologic

“Improving Care and Collaboration for Children with Neurologic and Behavioral Conditions” Webinar #1 Depression January 25 th, 2016 1: 00 pm -2: 00 pm

Kristi Kleinschmit, MD Dr. Kleinschmit is an Assistant Professor, in the Department of Psychiatry, Division of Child Psychiatry, and is the medical director of the Teenscope and Kidstar day treatment programs at the University of Utah Neuropsychiatric Institute. She is also the program director for the Triple Board and Child Psychiatry Residencies. Dr. Kleinschmit graduated from Tulane University School of Medicine in New Orleans, Louisiana. She completed a Triple Board residency at the University of Utah School of Medicine. She holds American Board certifications in Pediatrics, Adult Psychiatry, and Child and Adolescent Psychiatry. kristi. kleinschmit@hsc. utah. edu

Shawn Kohler, MD Dr. Kohler is a child and adolescent psychiatry second year fellow at the University of Utah. He is planning on continuing as an outpatient Child psychiatrist with the University in fall this year. He completed medical school at University of Illinois at Champaign Urbana. Shawn. kohler@hsc. utah. edu

Disclosures • Funding from: nothing to disclose • Institutional support from: nothing to disclose • I will be discussing off-label use of medications

CME Credit Accreditation: This activity has been planned and implemented in accordance with the essential areas and policies of the Accreditation Council for Continuing Medical Education through the joint providership of Primary Children’s Hospital, the Department of Pediatrics at the University of Utah School of Medicine, and UPIQ. Primary Children’s Hospital is accredited by the ACCME to provide continuing medical education for physicians. AMA Credit: Primary Children’s Hospital Designates this live activity for a maximum of 8 AMA PRA Category • 1 Credit(s)™. Physicians should only claim the credit that commensurate with the extent of their participation in the activity.

Disclaimer Most of the information today is based on clinical practice of myself and my colleagues, based on evidence when available, but also gleaning from the “art” of psychiatry. I am using brand names at times for clarification, not to promote any specific one.

Objectives • Update on antidepressant options in children/adolescents, including strategies and evidence for switching therapies • Treatment-resistant depression • Referrals to therapists

Antidepressant Classes SSRI’s – Fluoxetine, escitalopram, sertraline, paroxetine, fluvoxamine SNRI’s – duloxetine, venlafaxine, desvenlafaxine Other – buproprion, mirtazapine, trazodone, serzone MAOIs – tranylcypromine, phenelzine, isocarboxzid, selegiline transdermal TCAs – amitriptyline, desipramine, clomipramine, nortriptyline
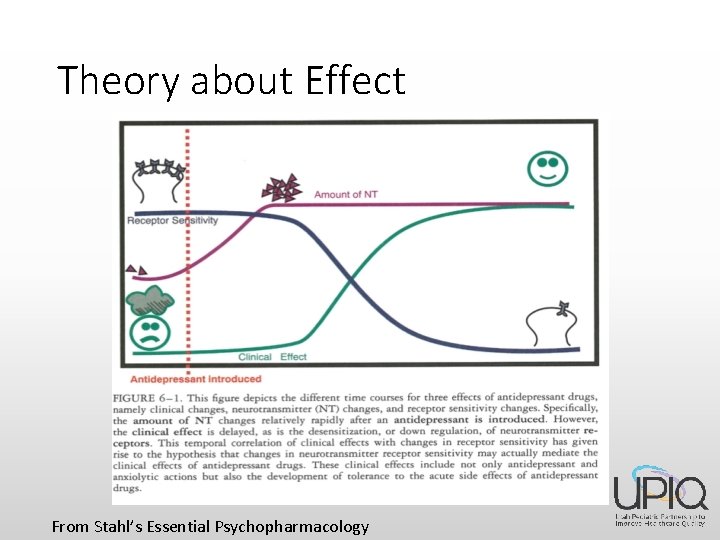
Theory about Effect From Stahl’s Essential Psychopharmacology

Selective Serotonin Reuptake Inhibitors (SSRI’s) Fluoxetine (Prozac) Escitalopram (Lexapro) Sertraline (Zoloft) Citalopram (Celexa) Fluvoxamine (Luvox) Paroxetine (Paxil)
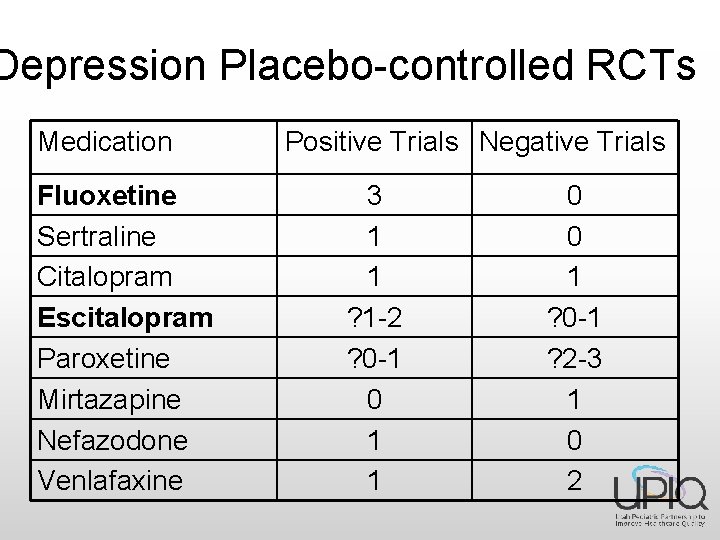
Depression Placebo-controlled RCTs Medication Fluoxetine Sertraline Citalopram Escitalopram Paroxetine Mirtazapine Nefazodone Venlafaxine Positive Trials Negative Trials 3 1 1 ? 1 -2 ? 0 -1 0 1 1 0 0 1 ? 0 -1 ? 2 -3 1 0 2
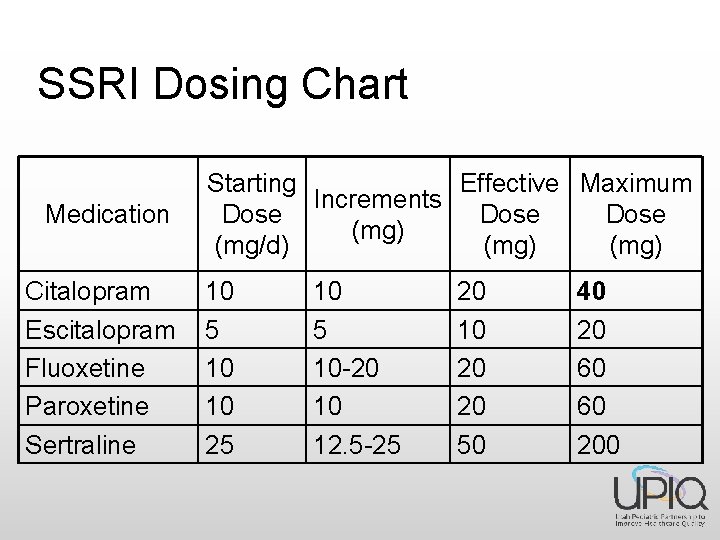
SSRI Dosing Chart Medication Citalopram Escitalopram Fluoxetine Paroxetine Sertraline Starting Effective Maximum Increments Dose (mg) (mg/d) (mg) 10 5 10 10 25 10 -20 10 12. 5 -25 20 10 20 20 50 40 20 60 60 200

SSRI – side effects EPS (either by self or in combo with neuroleptics) Suicidality Serotonin syndrome Activation - akathisia Mania – 5 -6% in some studies. Apathy / frontal lobe syndrome – mistaken for residual depression or under recognized • Sleep – fluoxetine = Increase REM latency, awakenings, decreased sleep efficiency & REM sleep. ? Medicine or depression effect? • Bleeding -? sertraline • Discontinuation – dizziness, movement disorder, paresthesias, nausea, headache, visual changes • • •
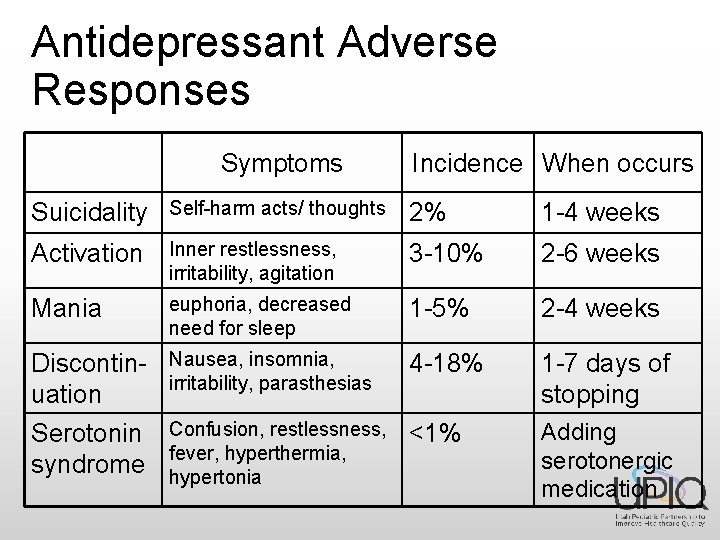
Antidepressant Adverse Responses Symptoms Incidence When occurs Suicidality Self-harm acts/ thoughts 2% 1 -4 weeks Activation Inner restlessness, irritability, agitation 3 -10% 2 -6 weeks Mania euphoria, decreased need for sleep 1 -5% 2 -4 weeks Discontinuation Serotonin syndrome Nausea, insomnia, irritability, parasthesias 4 -18% 1 -7 days of stopping Confusion, restlessness, fever, hyperthermia, hypertonia <1% Adding serotonergic medication
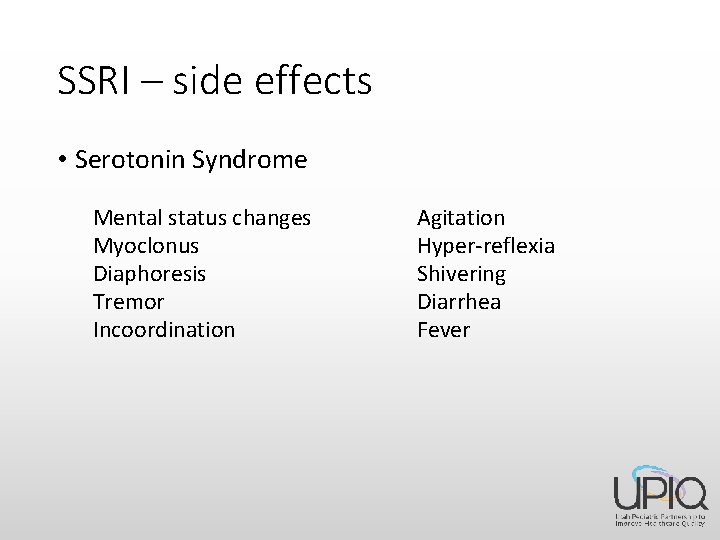
SSRI – side effects • Serotonin Syndrome Mental status changes Myoclonus Diaphoresis Tremor Incoordination Agitation Hyper-reflexia Shivering Diarrhea Fever
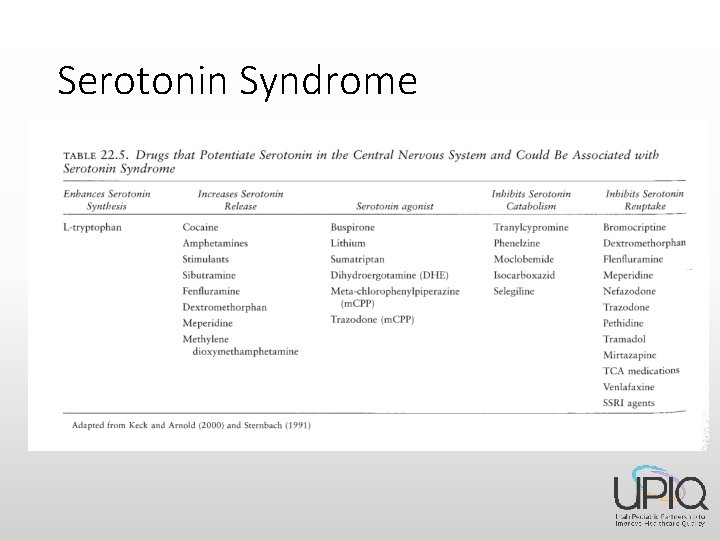
Serotonin Syndrome
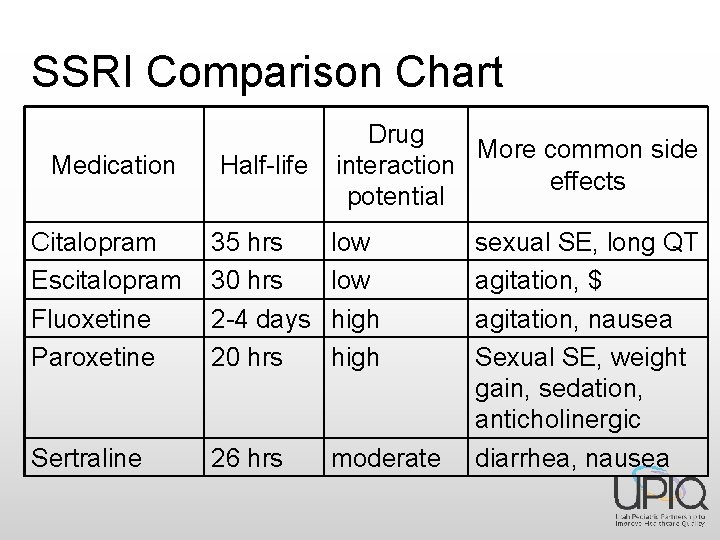
SSRI Comparison Chart Drug More common side interaction effects potential Medication Half-life Citalopram Escitalopram Fluoxetine Paroxetine 35 hrs 30 hrs 2 -4 days 20 hrs low high Sertraline 26 hrs moderate sexual SE, long QT agitation, $ agitation, nausea Sexual SE, weight gain, sedation, anticholinergic diarrhea, nausea
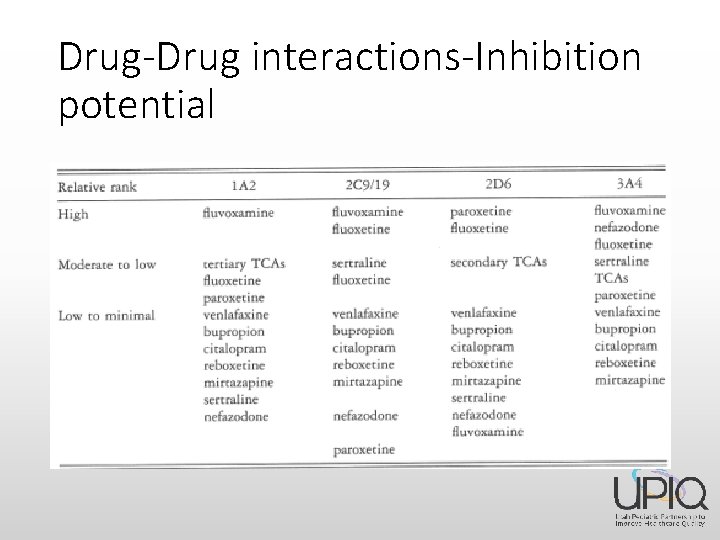
Drug-Drug interactions-Inhibition potential

Antidepressants and Suicidality • Black Box Warning (2004) • Warning of increased risk of suicidality in pediatric pts taking antidepressants. • FDA Analysis of short-term RCTs • Average risk of spontaneous suicidal thinking / behavior on drug was 4% vs. 2% on placebo • Toxicology studies • 0 -6% of suicides had antidepressants in blood • 25% had active prescriptions for antidepressants • Epidemiological Studies • Regional increases in SSRI use associated with decreases in youth suicide rates

SSRI Prescription Rates in the US, 2002 -2005, stratified by age group Ref: Gibbons, Am J Psych (2007)
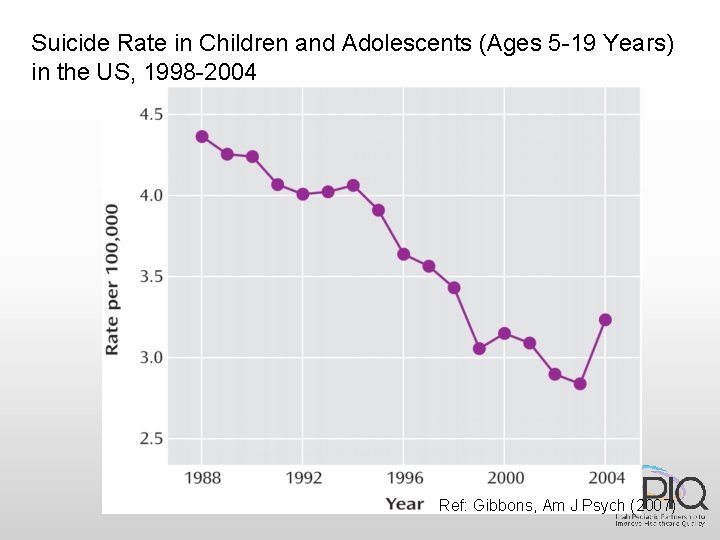
Suicide Rate in Children and Adolescents (Ages 5 -19 Years) in the US, 1998 -2004 Ref: Gibbons, Am J Psych (2007)
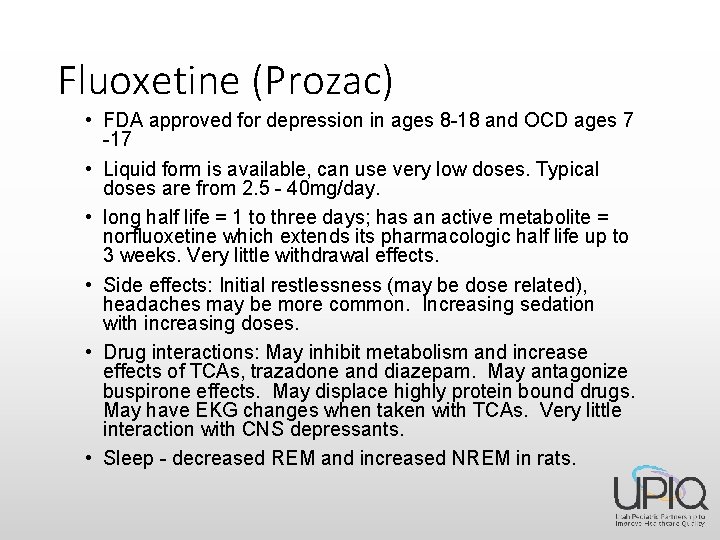
Fluoxetine (Prozac) • FDA approved for depression in ages 8 -18 and OCD ages 7 -17 • Liquid form is available, can use very low doses. Typical doses are from 2. 5 - 40 mg/day. • long half life = 1 to three days; has an active metabolite = norfluoxetine which extends its pharmacologic half life up to 3 weeks. Very little withdrawal effects. • Side effects: Initial restlessness (may be dose related), headaches may be more common. Increasing sedation with increasing doses. • Drug interactions: May inhibit metabolism and increase effects of TCAs, trazadone and diazepam. May antagonize buspirone effects. May displace highly protein bound drugs. May have EKG changes when taken with TCAs. Very little interaction with CNS depressants. • Sleep - decreased REM and increased NREM in rats.
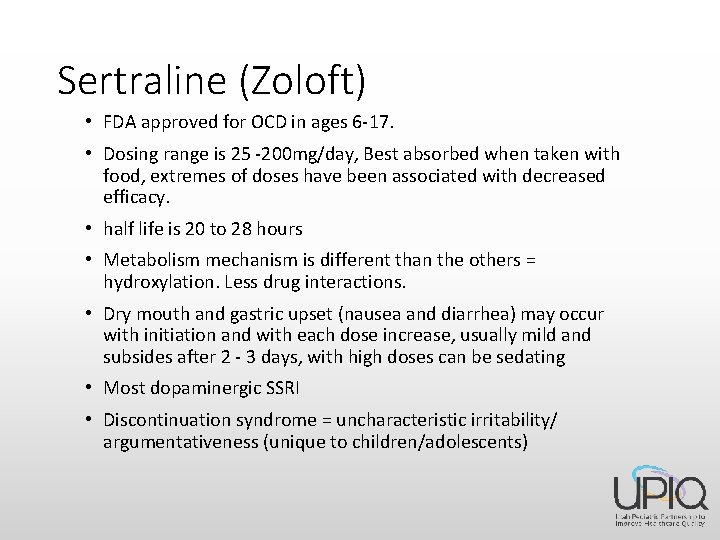
Sertraline (Zoloft) • FDA approved for OCD in ages 6 -17. • Dosing range is 25 -200 mg/day, Best absorbed when taken with food, extremes of doses have been associated with decreased efficacy. • half life is 20 to 28 hours • Metabolism mechanism is different than the others = hydroxylation. Less drug interactions. • Dry mouth and gastric upset (nausea and diarrhea) may occur with initiation and with each dose increase, usually mild and subsides after 2 - 3 days, with high doses can be sedating • Most dopaminergic SSRI • Discontinuation syndrome = uncharacteristic irritability/ argumentativeness (unique to children/adolescents)

Escitalopram (Lexapro) • FDA approved for depression in ages 12 -17 • Isomer form of citalopram. • Touted as having less side effects and better tolerated than citalopram • Effectively concentrated citalopram = twice as strong. • Dose: Start 5 mg Qday, increase by 5 mg q 2 weeks, max dose 20 mg
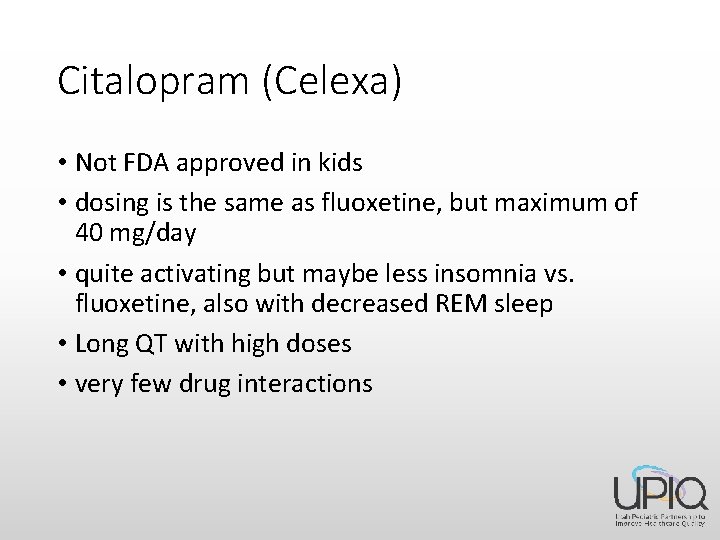
Citalopram (Celexa) • Not FDA approved in kids • dosing is the same as fluoxetine, but maximum of 40 mg/day • quite activating but maybe less insomnia vs. fluoxetine, also with decreased REM sleep • Long QT with high doses • very few drug interactions

Vilazadone (Viibryd) • Selectively inhibits serotonin reuptake and partially agonizes serotonin 5 -HT 1 A receptors • No studies in kids, not FDA approved • $150 -176 per 30 tabs

Serotonin Norepinephrine Reuptake Inhibitors (SNRI’s) • Venlafaxine (Effexor) • Duloxetine (Cymbalta) • Approved for GAD in teens • Desvenlafaxine (Pristiq)
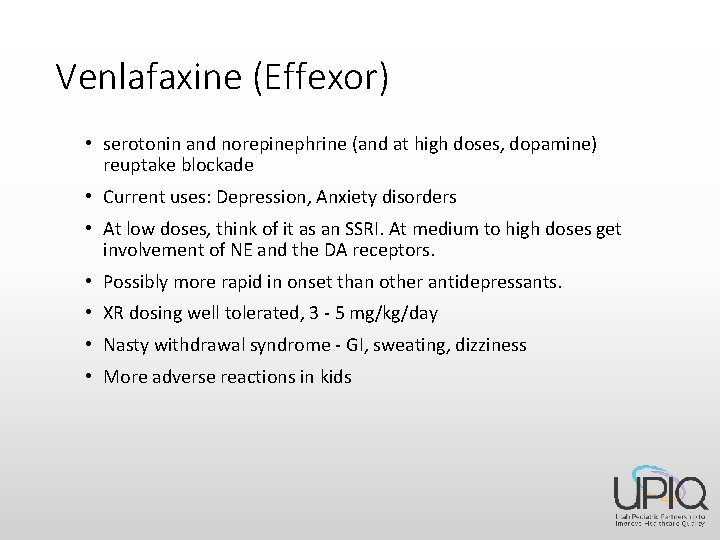
Venlafaxine (Effexor) • serotonin and norepinephrine (and at high doses, dopamine) reuptake blockade • Current uses: Depression, Anxiety disorders • At low doses, think of it as an SSRI. At medium to high doses get involvement of NE and the DA receptors. • Possibly more rapid in onset than other antidepressants. • XR dosing well tolerated, 3 - 5 mg/kg/day • Nasty withdrawal syndrome - GI, sweating, dizziness • More adverse reactions in kids

Desvenlafaxine (Pristiq) • • New isomer form of venlafaxine No data in kids $130 per month Would not recommend using

Duloxetine (Cymbalta) • Most like a TCA of the SNRIs • TCAs have been shown not to be effective in children and adolescents secondary to the immaturity of the noradrenergic system. • Limited data in youth • Just became generic
Bupropion (Wellbutrin) • norepinephrine and dopamine reuptake inhibitor • No approved uses < 18 y/o • Current uses : Depression, ADHD, smoking cessation • Contraindications - seizure disorder, current or prior diagnosis of bulimia or anorexia, history of closed head injury • Side effects: stimulating (last dose should be before 3 - 4 PM), insomnia, agitation, rare GI symptoms • no sexual dysfunction - can add to SSRI and reverse SSRI sexual side effects or to boost efficacy • consider for treatment of depression in people who have history of dopamine seeking behaviors - thrill seekers, stimulant/cocaine abusers, cigarette users • seizure incidence= 1 -4/1000 at normal doses

Treatment Resistant Depression
Switching Antidepressants • Limited data in kids or adults • Kudlow 2014, reviewed evidence of early improvement to predict final response (28 studies) and did lit review of RCT’s for switching (only 3). ONLY ADULT STUDIES. • Contradictory data, but felt comfortable recommending change in management if no response at 4 weeks (increase dose, new medication, augmentation)
Treatment: TORDIA Study • 12 week RCT conducted at 6 clinical sites • 334 patients with MDD, ages 12 -18 • Had not responded to 2 -month initial treatment with an SSRI. • Randomized to 4 treatment strategies • Switch to different SSRI (paraxotine / citalopram or fluoxetine) • Switch to different SSRI + CBT • Switch to venlafaxine + CBT Brent, JAMA (2008); Asarnow, J Am Acad Child (2009)

TORDIA Study Results • CBT + switch to either medication regimen showed a higher response rate • No difference in response rate between venlafaxine and a second SSRI • Treatment with venlafaxine resulted in more side effects and less robust response with severe depression and SI • Poorer response predicted by severity, SI, substance abuse, sleeping medication, and family conflict
TORDIA at Week 24 • At week 12, responders continued, non-responders were given CBT + med x 12 more weeks • 38. 9% achieved remission • Likelihood of remission higher and faster if responded by week 12. • At week 6, participants who eventually remitted showed 48% reduction in scales • Augmentation of non-responders within 12 weeks with therapy or mood stabilizer predicted remission
How to treat resistant depression? • Monitor response, and increase dose or switch if none by 6 weeks (or even 4? ) • Ensure adherance to meds • Switch to venlafaxine in kids no better than switch to a different SSRI (unlike adult data) • Augment with therapy (if not already doing) • Relook at diagnoses and comorbidities • Refer to psychiatry • ECT, TMS, adjunctive medications
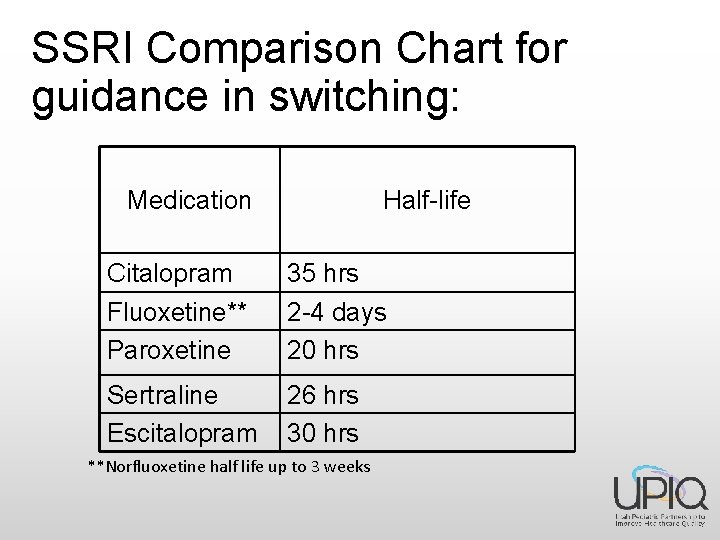
SSRI Comparison Chart for guidance in switching: Medication Half-life Citalopram Fluoxetine** Paroxetine 35 hrs 2 -4 days 20 hrs Sertraline Escitalopram 26 hrs 30 hrs **Norfluoxetine half life up to 3 weeks

Final words on the magic of choosing an antidepressant: • Gather family history. Use what works for other family members • If no family history of SSRI use, go with the FDA approvals or side effect profile. • If still in doubt:

• Just pick one • If that doesn’t work, pick another one • If that still doesn’t work, and they are at good doses for good amounts of time, feel free to refer or get a second opinion. Or add therapy!

Therapists • Don’t only give a name or two, because those folks are usually booked out • Use insurance panel as guide • Have family call therapist-should take time to talk over the phone a little. Things to ask: • Population works with? (CBT with children/teens) • Family involvement • Availability • While waiting, refer to various on-line help sites that were discussed in initial session.

References • Brent D et al. Switching to another SSRI or to venlafaxine with or without cognitive behavioral therapy for adolescents with SSRI-resistant depression. JAMA. 2008; 299 (8): 901 -913. • Emslie GJ et al. Treatment of resistant depression in adolescents (TORDIA): week 24 outcomes. Am J Psychiatry. 2010; 167(7): 782 -791. • Kudlow PA, Mc. Intyre RS, Lam RW. Early switching strategies in antidepressant non-responsders: current evidence and future research directions. CNS Drugs. 2014

Comments/Questions Reminder: 2 nd Team Lead Call, Tuesday, February 21 rd @ 1: 00 pm
- Slides: 43