IMPROVING AND OPTIMIZING EARLY INFANT DIAGNOSIS IAS 2019













- Slides: 38

IMPROVING AND OPTIMIZING EARLY INFANT DIAGNOSIS IAS 2019 21 -24 July 2019 1| Fausta Mosha: MD, PHD Laboratory Technical Officer – HIV/TB/Hepatitis WHO/AFRO

Presentation Outline § Indeterminate range § Confirmatory test § SOP for discordant results § EID algorithm 2|

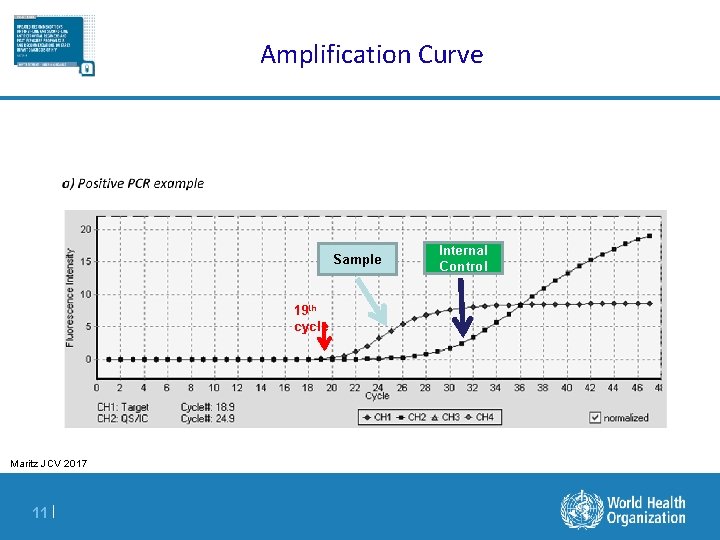
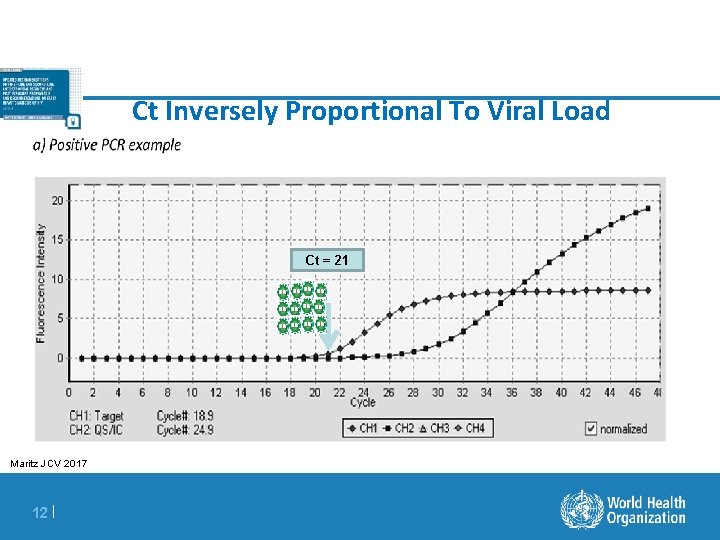
Definition Of Terms For Diagnostic Results § Positive Predictive value (PPV): the probability that an infant with a positive EID test result is HIV infected § Indeterminate range: A range of viral copy equivalents that would be too low to accurately diagnose as positive § Indeterminate results have a detectable target, as determined by the instrument, but the amplified viral signal is of such a low level that it could potentially be a false-positive result § False-positive result: HIV-uninfected infants incorrectly identified as HIV-infected § False-negative result: HIV-infected infants incorrectly identified as HIV-uninfected § Non-negative result: Any positive or indeterminate result § Discordant result: positive/detectable first test; negative second test § Cycle threshold ( Ct) : the point at which virus amplification is first observed during the course of repeated PCR cycles – The cycle threshold is inversely correlated to the amount of virus in the sample 3|

Rationale For This Technical Update A. Reduced MTCT rates – scale up of treat all policy in pregnant and breastfeeding women i. Reduced viremia in infants ii. Decrease in positive predictive value of nucleic acid testing (NAT) iii. Lower specificity and higher proportion of false-positive test results B. Implementation of Enhanced Postnatal prophylaxis (e. PNP) i. 4| Delays in antibody development affects RDT use and interpretation

INDETERMINATE RANGE 5|

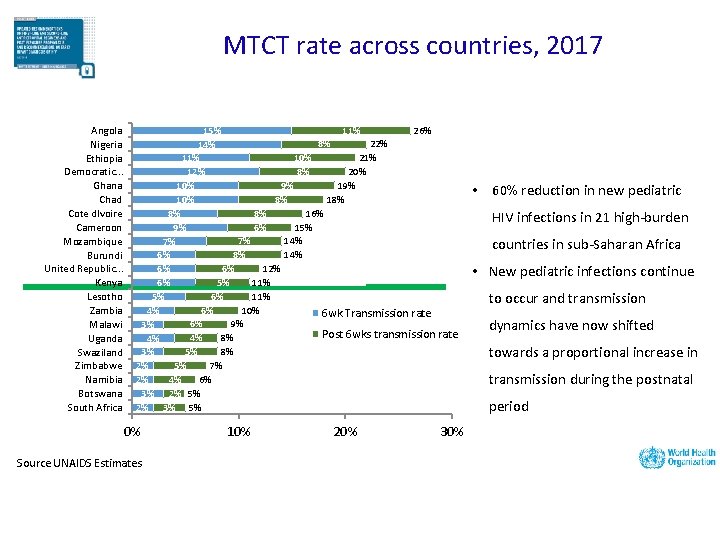
MTCT rate across countries, 2017 Angola Nigeria Ethiopia Democratic. . . Ghana Chad Cote d. Ivoire Cameroon Mozambique Burundi United Republic. . . Kenya Lesotho Zambia Malawi Uganda Swaziland Zimbabwe Namibia Botswana South Africa 11% 26% 15% 8% 22% 14% 10% 21% 11% 8% 20% 12% 9% 10% 8% 16% 8% 6% 15% 9% 7% 14% 7% 8% 14% 6% 6% 12% 6% 5% 11% 6% 6% 11% 5% 6% 10% 4% 6 wk Transmission rate 6% 9% 3% Post 6 wks transmission rate 4% 8% 4% 5% 8% 3% 5% 7% 2% 4% 6% 2% 3% 2% 5% 2% 3% 5% 0% Source UNAIDS Estimates 10% 20% 30% • 60% reduction in new pediatric HIV infections in 21 high-burden countries in sub-Saharan Africa • New pediatric infections continue to occur and transmission dynamics have now shifted towards a proportional increase in transmission during the postnatal period

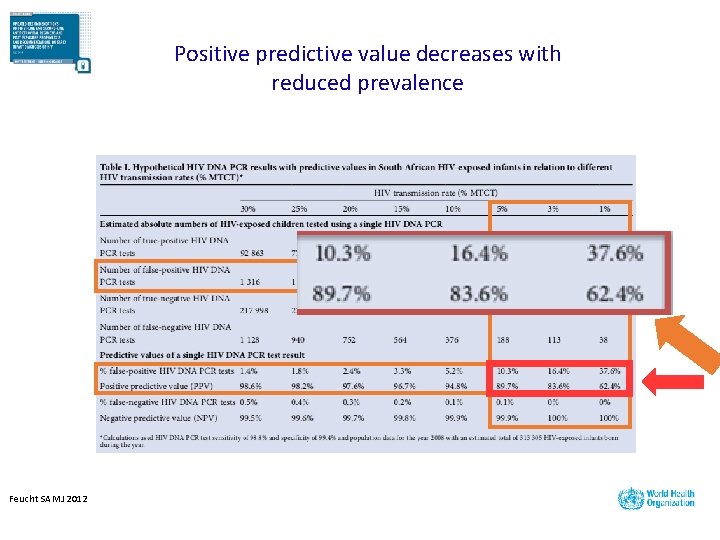
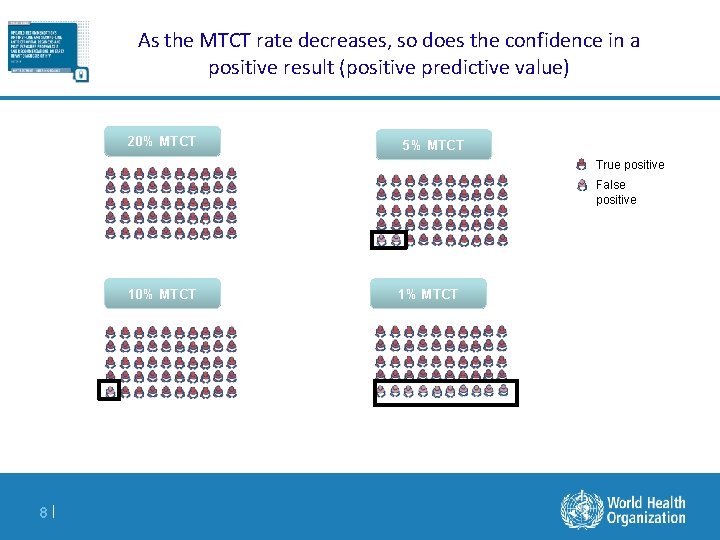
Positive predictive value decreases with reduced prevalence Feucht SAMJ 2012

As the MTCT rate decreases, so does the confidence in a positive result (positive predictive value) 20% MTCT 5% MTCT True positive False positive 10% MTCT 8| 1% MTCT

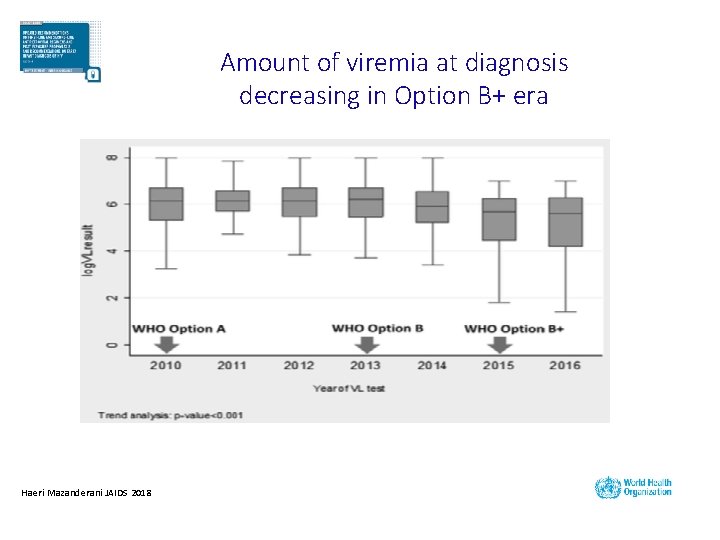
Amount of viremia at diagnosis decreasing in Option B+ era Haeri Mazanderani JAIDS 2018

Several technologies are available for EID testing Qualitative technologies generally report ‘detected’ or ‘not detected’, which gets understood as ‘positive’ or ‘negative’, respectively. All tests with WHOPQ have high specificity > 98%

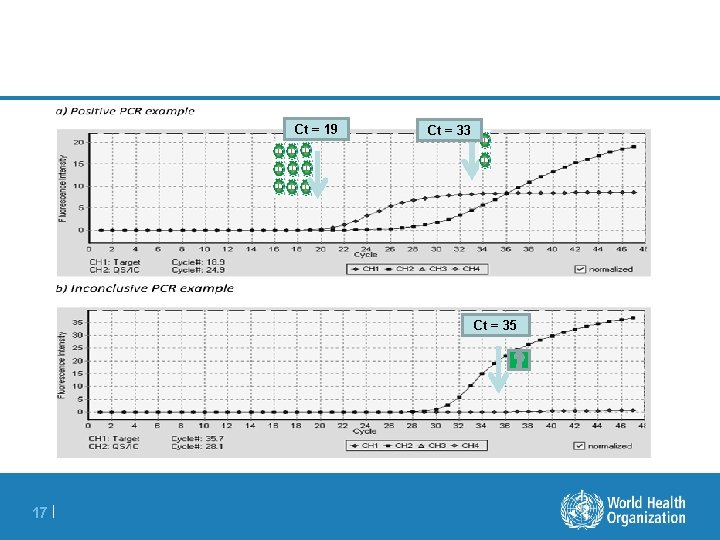
Amplification Curve Sample 19 th cycle Maritz JCV 2017 11 | Internal Control

Ct Inversely Proportional To Viral Load Ct = 21 Maritz JCV 2017 12 |

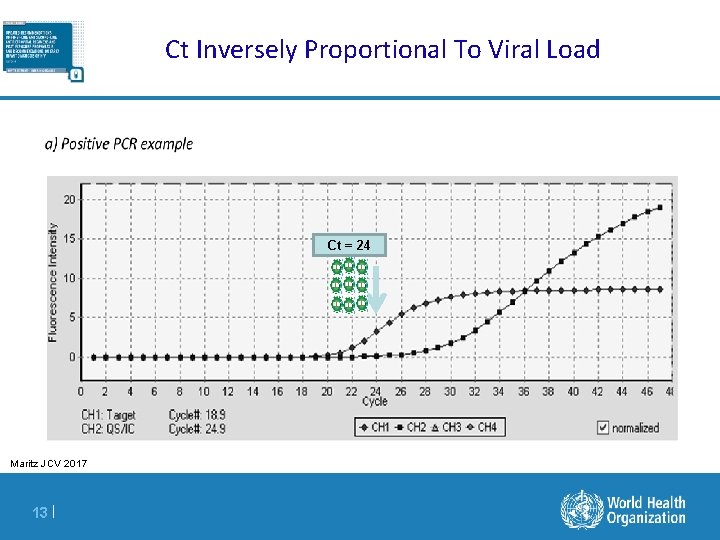
Ct Inversely Proportional To Viral Load Ct = 24 Maritz JCV 2017 13 |

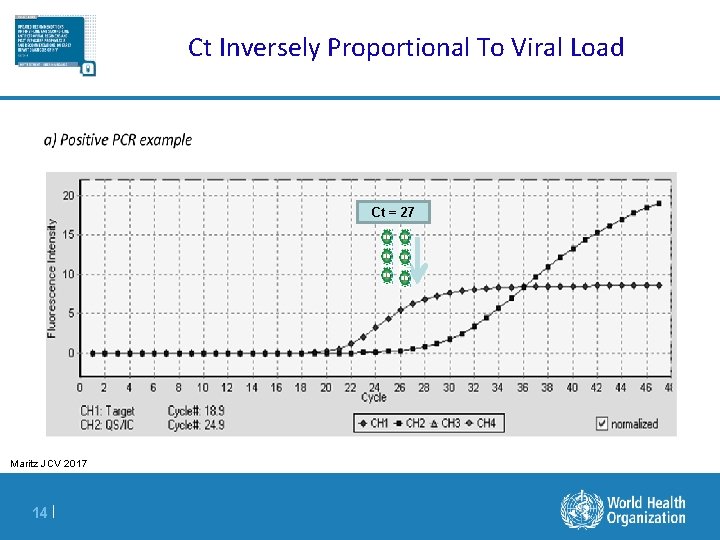
Ct Inversely Proportional To Viral Load Ct = 27 Maritz JCV 2017 14 |

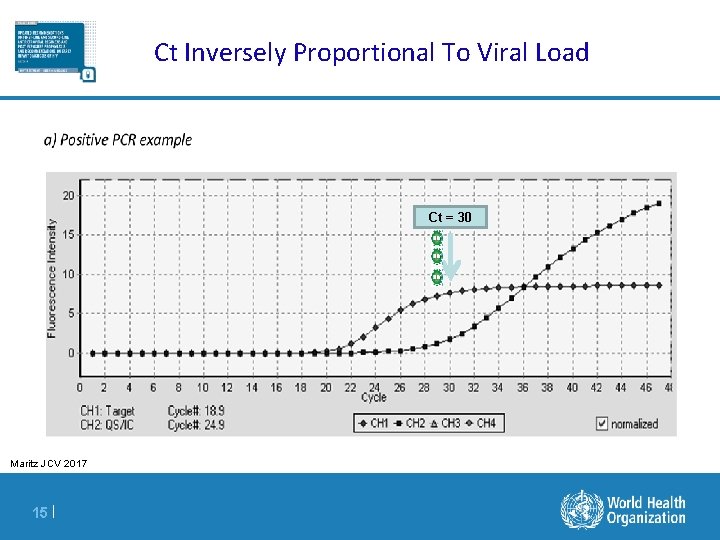
Ct Inversely Proportional To Viral Load Ct = 30 Maritz JCV 2017 15 |

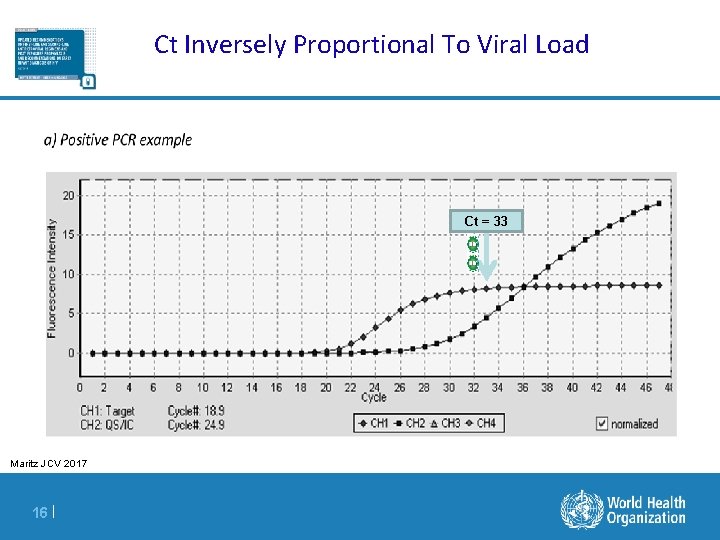
Ct Inversely Proportional To Viral Load Ct = 33 Maritz JCV 2017 16 |

Ct = 19 Ct = 33 Ct = 35 17 |

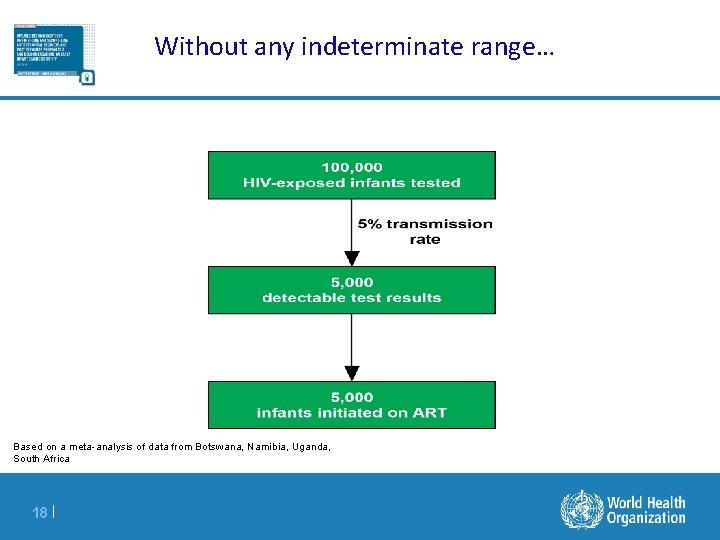
Without any indeterminate range… Based on a meta-analysis of data from Botswana, Namibia, Uganda, South Africa 18 |

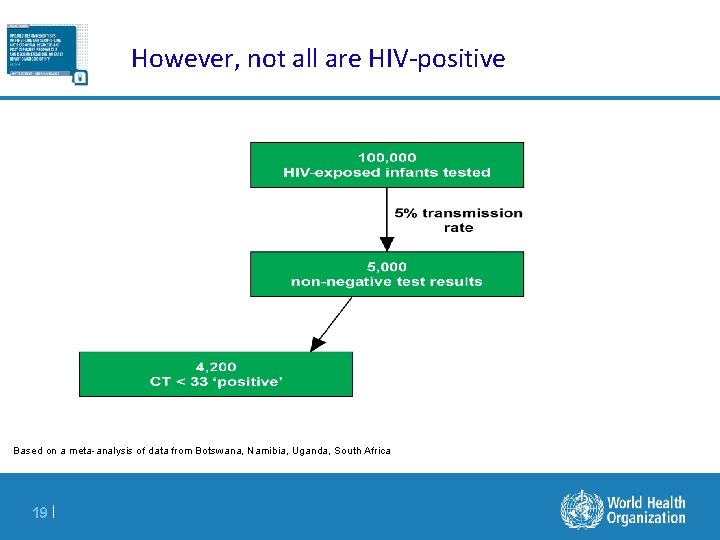
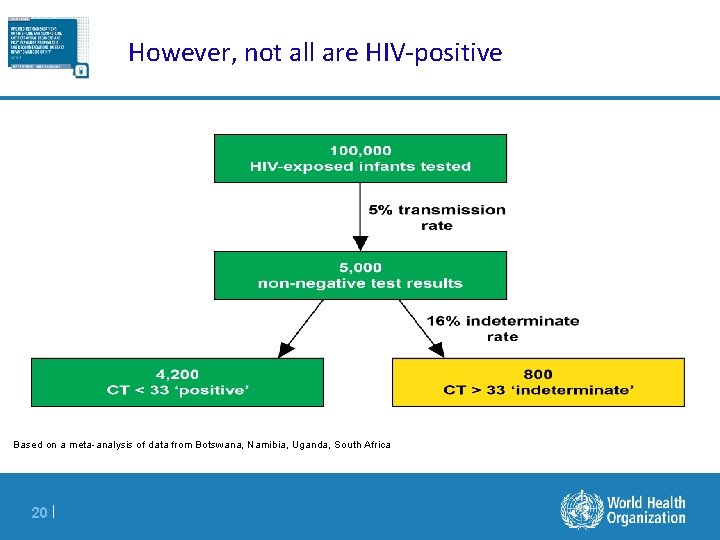
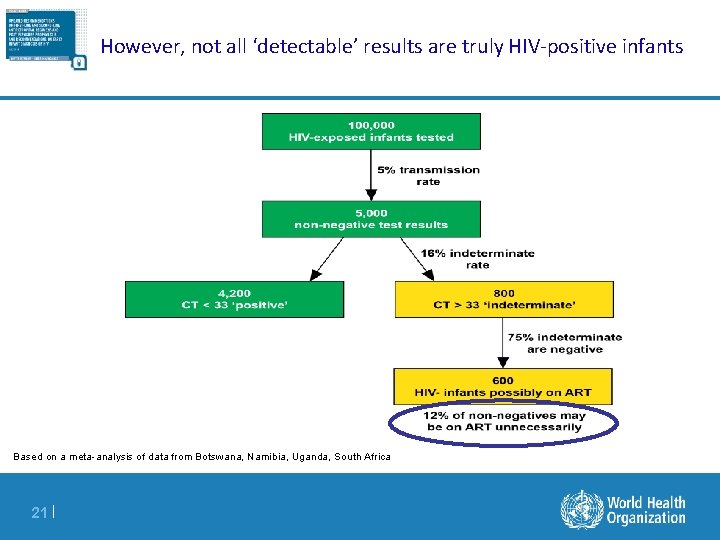
However, not all are HIV-positive Based on a meta-analysis of data from Botswana, Namibia, Uganda, South Africa 19 |

However, not all are HIV-positive Based on a meta-analysis of data from Botswana, Namibia, Uganda, South Africa 20 |

However, not all ‘detectable’ results are truly HIV-positive infants Based on a meta-analysis of data from Botswana, Namibia, Uganda, South Africa 21 |

New WHO Recommendation Qualitative technologies generally report ‘detected’ or ‘not detected’, which is interpreted as ‘positive’ or ‘negative’, respectively. THEREFORE Identifying and repeat testing samples with low level viremia by introducing an indeterminate range can minimize unnecessary treatment initiation. 22 |

SOP for managing INDETERMINATE test results § All indeterminate tests should be repeat tested on the same specimen, if and when available § If the same specimen cannot be repeat tested, then a new specimen should be requested and tested as quickly as possible § For specimens with two indeterminate test results, a new specimen should be requested § For infants repeatedly testing indeterminate, it is suggested that a team of experts review clinical and test information to determine the best followup care 23 |

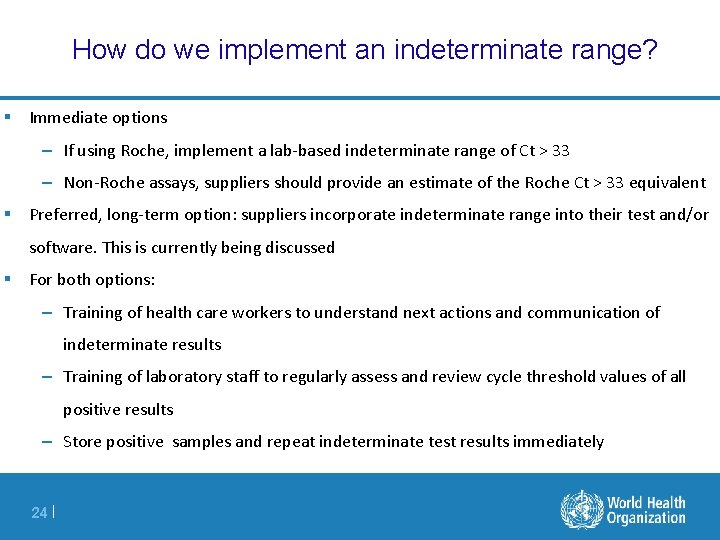
How do we implement an indeterminate range? § Immediate options – If using Roche, implement a lab-based indeterminate range of Ct > 33 – Non-Roche assays, suppliers should provide an estimate of the Roche Ct > 33 equivalent § Preferred, long-term option: suppliers incorporate indeterminate range into their test and/or software. This is currently being discussed § For both options: – Training of health care workers to understand next actions and communication of indeterminate results – Training of laboratory staff to regularly assess and review cycle threshold values of all positive results – Store positive samples and repeat indeterminate test results immediately 24 |

CONFIRMATORY TEST 25 |

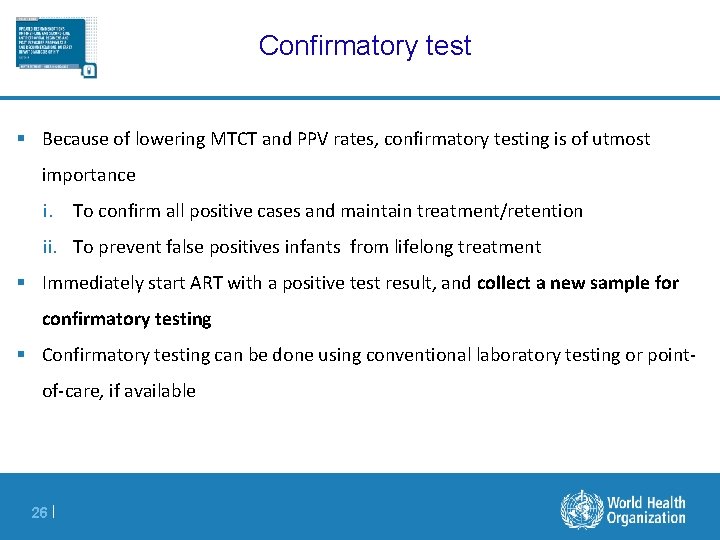
Confirmatory test § Because of lowering MTCT and PPV rates, confirmatory testing is of utmost importance i. To confirm all positive cases and maintain treatment/retention ii. To prevent false positives infants from lifelong treatment § Immediately start ART with a positive test result, and collect a new sample for confirmatory testing § Confirmatory testing can be done using conventional laboratory testing or pointof-care, if available 26 |

MANAGING DISCORDANT RESULTS 27 |

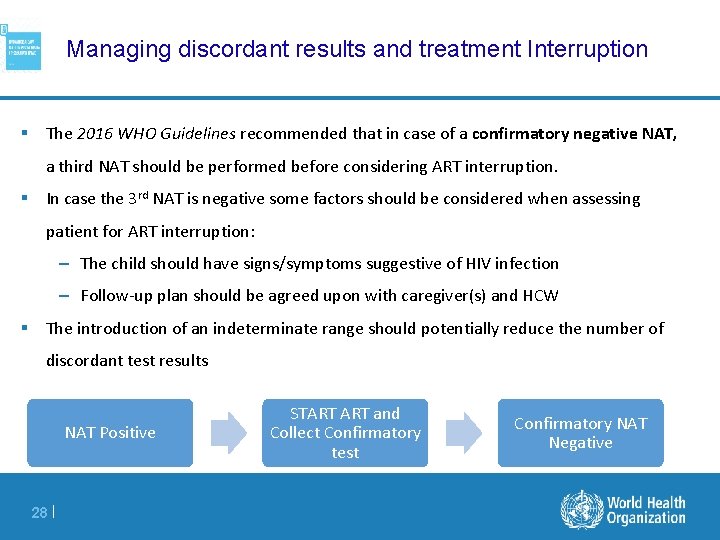
Managing discordant results and treatment Interruption § The 2016 WHO Guidelines recommended that in case of a confirmatory negative NAT, a third NAT should be performed before considering ART interruption. § In case the 3 rd NAT is negative some factors should be considered when assessing patient for ART interruption: – The child should have signs/symptoms suggestive of HIV infection – Follow-up plan should be agreed upon with caregiver(s) and HCW § The introduction of an indeterminate range should potentially reduce the number of discordant test results NAT Positive 28 | START and Collect Confirmatory test Confirmatory NAT Negative

Factors to consider follow up of infant undergoing treatment interruption § Active follow-up to ensure that a potentially infected infant is retained and reinitiated on treatment if virological rebound occurs § Virological rebound is expected to happen within 8 months of interruption in >99% of HIV-infected infants 1 § Infants who develop signs and symptoms indicative of HIV infection should undergo immediate NAT testing § Breastfeeding and continued risk of transmission require follow-up and appropriate testing throughout the period of risk until final diagnosis 1 Avy Violari et al. Time to viral rebound after stopping ART in children treated from infancy in CHER. Abstract #137, CROI; March 4 -7, 2018 29 |

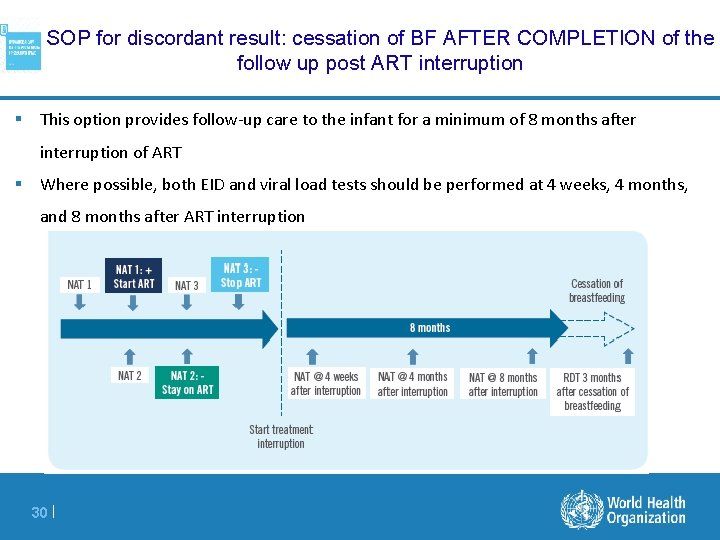
SOP for discordant result: cessation of BF AFTER COMPLETION of the follow up post ART interruption § This option provides follow-up care to the infant for a minimum of 8 months after interruption of ART § Where possible, both EID and viral load tests should be performed at 4 weeks, 4 months, and 8 months after ART interruption 30 |

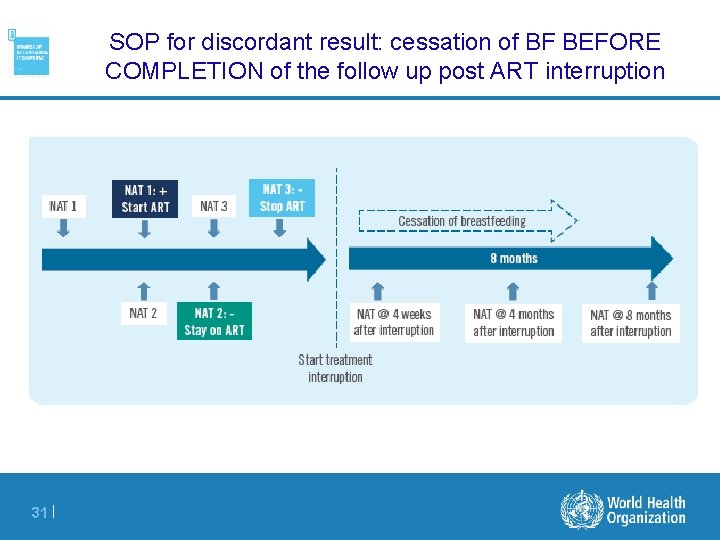
SOP for discordant result: cessation of BF BEFORE COMPLETION of the follow up post ART interruption 31 |

EID ALGORITHM 32 |

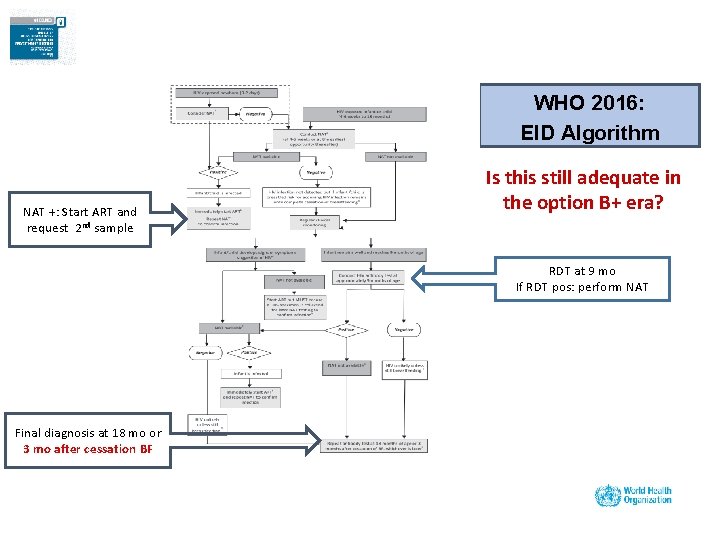
WHO 2016: EID Algorithm NAT +: Start ART and request 2 nd sample Is this still adequate in the option B+ era? RDT at 9 mo If RDT pos: perform NAT Final diagnosis at 18 mo or 3 mo after cessation BF

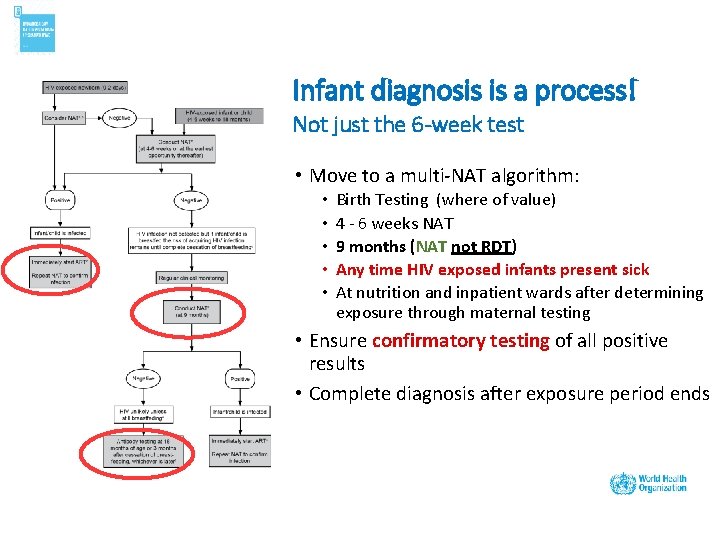
Infant diagnosis is a process! Not just the 6 -week test • Move to a multi-NAT algorithm: • • • Birth Testing (where of value) 4 - 6 weeks NAT 9 months (NAT not RDT) Any time HIV exposed infants present sick At nutrition and inpatient wards after determining exposure through maternal testing • Ensure confirmatory testing of all positive results • Complete diagnosis after exposure period ends

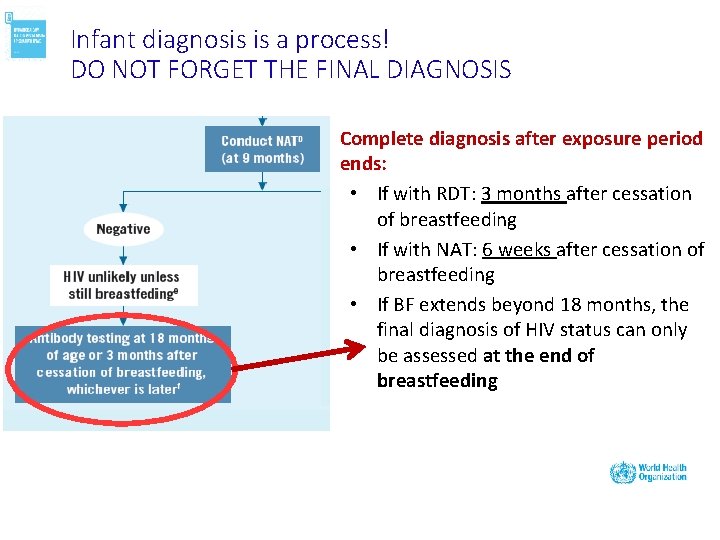
Infant diagnosis is a process! DO NOT FORGET THE FINAL DIAGNOSIS Complete diagnosis after exposure period ends: • If with RDT: 3 months after cessation of breastfeeding • If with NAT: 6 weeks after cessation of breastfeeding • If BF extends beyond 18 months, the final diagnosis of HIV status can only be assessed at the end of breastfeeding

Moving forward… § The WHO 2018 updated recommendation enables access to more potent regimens and promote actions to ensure more accurate infant diagnosis. § Tools and guidance have already been developed to support country adoption and adaptation of these guidelines § Dedicated efforts are being planned to actively assist priority countries in their efforts to implement these recommendations § WHO continues to work with global partners to promote a collaborative and coordinated agenda that ensures innovation and acceleration for an AIDS FREE generation 36 |

Acknowledgment § Organizing team § WHO AFRO – CDs • HTH § WHO HQ 37 |

Thank you 38 |