Imported Vaccine Associated Paralytic Poliomyelitis Arizona 2005 Shoana













- Slides: 17

Imported Vaccine Associated Paralytic Poliomyelitis Arizona, 2005 Shoana Anderson Arizona Dept of Health Services March 7, 2006

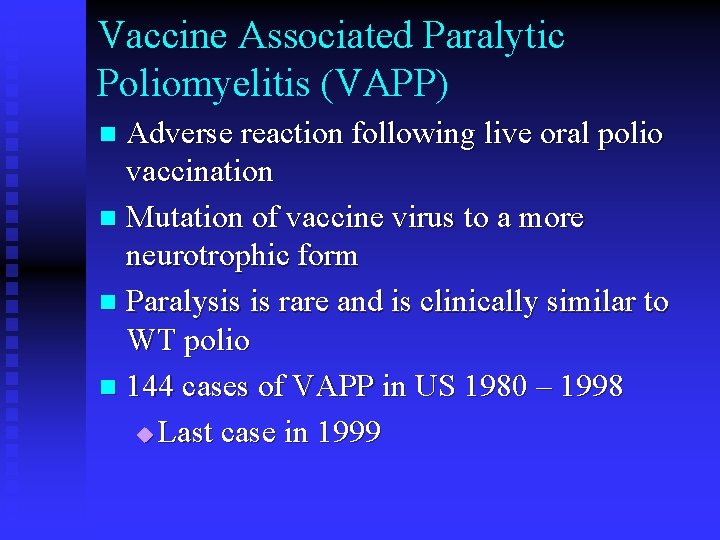
Vaccine Associated Paralytic Poliomyelitis (VAPP) Adverse reaction following live oral polio vaccination n Mutation of vaccine virus to a more neurotrophic form n Paralysis is rare and is clinically similar to WT polio n 144 cases of VAPP in US 1980 – 1998 u Last case in 1999 n

VAPP Increased risk for adults (>18 yrs) n Immunocompromised at increased risk n Higher risk associated with first dose VAPP in US (1980 – 1998)* u 41% Healthy OPV recipients u 31% Healthy contacts u 24% Immunodeficient u 5% Community acquired n * Centers for Disease Control and Prevention. Epidemiology and Prevention of Vaccine-Preventable Diseases. 2004

Suspect Polio? ? n 3/18/2005: ADHS notified of a suspect polio case in a 22 year old female u Bilateral lower extremity weakness u Previous difficulty breathing u High serologic titers to all 3 poliovirus types

Patient History Case had been residing in Costa Rica since early January with host family n Philosophically opposed to vaccination n Previously healthy n Travel to several locations in Central and South America n No insect bites n No ill contacts n

Clinical History 3/2/05: Patient reported fever, sore throat, and myalgia n 3/3/05: Developed headache and neck and back pain u Urinary retention, bilateral LE weakness n 3/6/05: Hospitalized in Costa Rica n 3/8/05: Air-evacuated to Phoenix hospital n

Clinical History Case developed SOB, difficulty speaking, and respiratory failure requiring intubation n Demonstrated ascending flaccid paralysis u Reflexes absent u No sensory dysfunction n Differentials: transverse myelitis, GBS, viral meningitis n

Clinical Findings and Diagnostic Studies CSF/blood: pleocytosis, elevated protein n Negative for: HIV, WNV, SLE, CMV, Dengue virus n MRI: T 2 signal hyperintensity n EMG: axonal polyneuropathy n

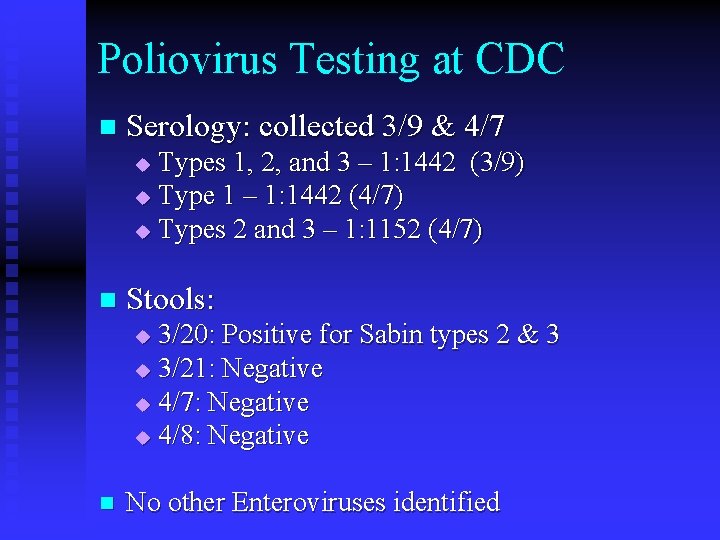
Poliovirus Testing at CDC n Serology: collected 3/9 & 4/7 Types 1, 2, and 3 – 1: 1442 (3/9) u Type 1 – 1: 1442 (4/7) u Types 2 and 3 – 1: 1152 (4/7) u n Stools: 3/20: Positive for Sabin types 2 & 3 u 3/21: Negative u 4/7: Negative u 4/8: Negative u n No other Enteroviruses identified

Clinical Course 60 Day Follow-up (May 2005) n n n Significant residual paralysis of both lower extremities u Primarily quads u Patient could ambulate with assistance u Areflexia No remaining respiratory complications or upper extremity weakness Classified as VAPP by committee: September , 2005

Exposure to OPV n Case resided with host family (no young children) u Son lived next door – frequent visits t 2 month old t 3 year old n 2 month old received first dose of OPV on 1/19/2005

Challenges Inexperience with poliovirus u Delays in testing u Inappropriate treatment u Investigation protocols and specimen collection n International investigation and coordination n Vaccination of household members u 2 younger siblings of the case only received 1 dose of IPV n

Missed Opportunities Case received HAV & HBV vaccination prior to travel n Unaware of the risk of travel to OPV-using country n

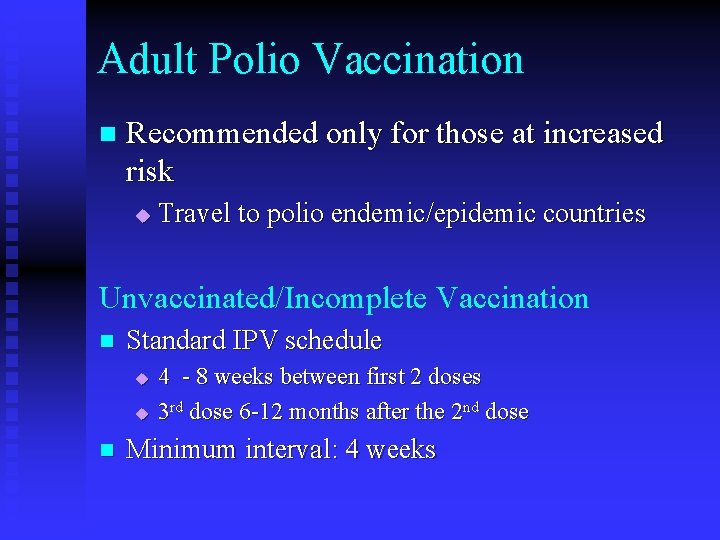
Adult Polio Vaccination n Recommended only for those at increased risk u Travel to polio endemic/epidemic countries Unvaccinated/Incomplete Vaccination n Standard IPV schedule u u n 4 - 8 weeks between first 2 doses 3 rd dose 6 -12 months after the 2 nd dose Minimum interval: 4 weeks

Conclusions First case of paralytic polio in the US since 1999 n First documented imported VAPP case n Exposed to OPV through vaccination of grandchild in host family n Consider vaccination of travelers to both polio endemic and OPV-using countries n

Arizona Dept of Health Services n n n Laura Erhart Susan Goodykoontz Karen Lewis Immunization Program David Engelthaler, State Epi Yavapai County Health Department n n Stephen Everett Sue Robyn St Joseph’s Hospital and Flagstaff Medical Center n n Kathy Howard Dr. Russell Walker Dr. Mark Lacy Dr. Vincent Stack CDC n n Jim Alexander Kristin Kenyan Lorraine Alexander Mark Pallansch

Questions Shoana Anderson Arizona Department of Health Services anderssm@azdhs. gov 602. 364. 3676
 Prevention and control of poliomyelitis
Prevention and control of poliomyelitis The most effective vaccine yet
The most effective vaccine yet Parasite
Parasite Prevention and control of poliomyelitis
Prevention and control of poliomyelitis Types of poliomyelitis
Types of poliomyelitis Hemo
Hemo Etiology of malaria
Etiology of malaria Lumbagon
Lumbagon False projection in paralytic squint
False projection in paralytic squint Jesus heals a paralytic
Jesus heals a paralytic Paralytic at bethesda
Paralytic at bethesda Market forms of meat cured meat
Market forms of meat cured meat Rabies vaccine dose
Rabies vaccine dose 7 rights of vaccine administration
7 rights of vaccine administration Zostavax reconstitution
Zostavax reconstitution Vaccine storage and handling sop worksheet
Vaccine storage and handling sop worksheet Edible rabies vaccine
Edible rabies vaccine Gudair vaccine gun
Gudair vaccine gun