Important reminder Midterm is Friday March 24 Chapters

- Slides: 26

Important reminder! Midterm is Friday, March 24 Chapters 5 -8 (except today’s material) 12/15/2021 1

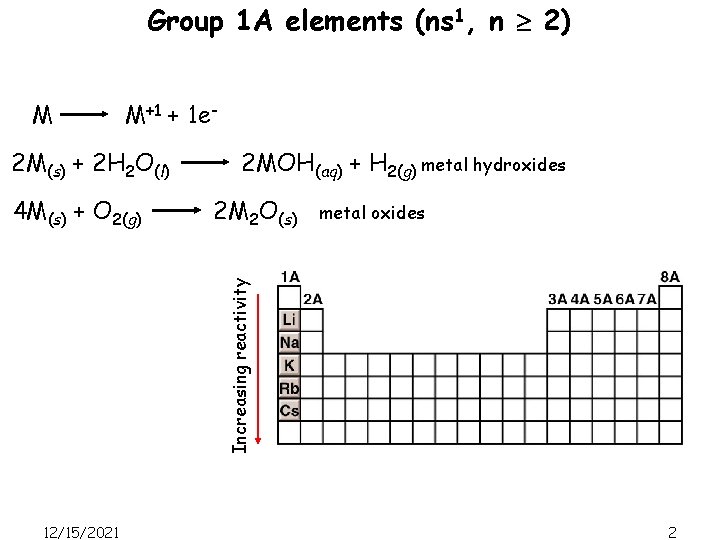
Group 1 A elements (ns 1, n 2) M M+1 + 1 e- 2 M(s) + 2 H 2 O(l) 2 M 2 O(s) metal oxides Increasing reactivity 4 M(s) + O 2(g) 2 MOH(aq) + H 2(g) metal hydroxides 12/15/2021 2

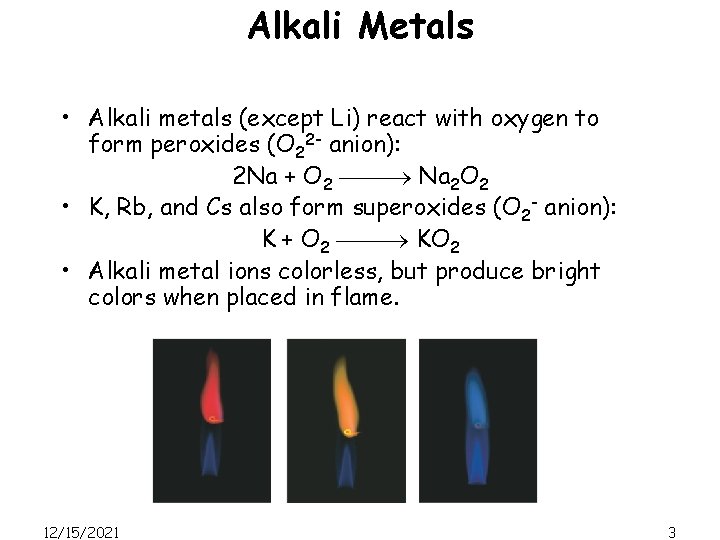
Alkali Metals • Alkali metals (except Li) react with oxygen to form peroxides (O 22 - anion): 2 Na + O 2 Na 2 O 2 • K, Rb, and Cs also form superoxides (O 2 - anion): K + O 2 KO 2 • Alkali metal ions colorless, but produce bright colors when placed in flame. 12/15/2021 3

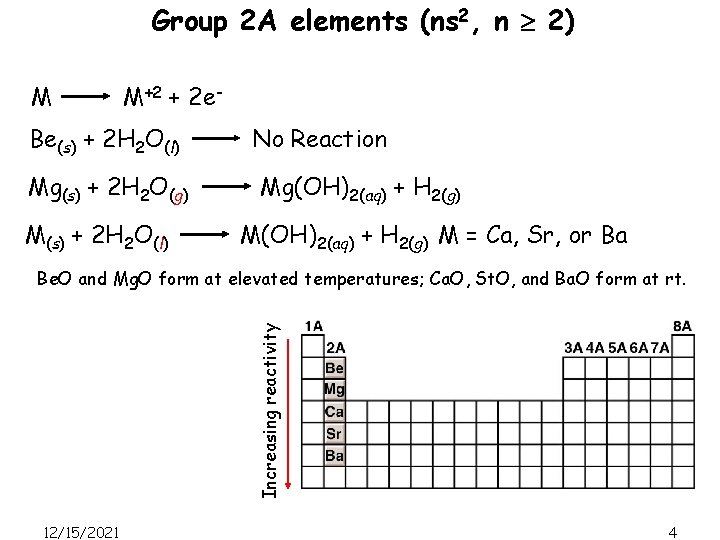
Group 2 A elements (ns 2, n 2) M M+2 + 2 e- Be(s) + 2 H 2 O(l) Mg(s) + 2 H 2 O(g) M(s) + 2 H 2 O(l) No Reaction Mg(OH)2(aq) + H 2(g) M = Ca, Sr, or Ba Increasing reactivity Be. O and Mg. O form at elevated temperatures; Ca. O, St. O, and Ba. O form at rt. 12/15/2021 4

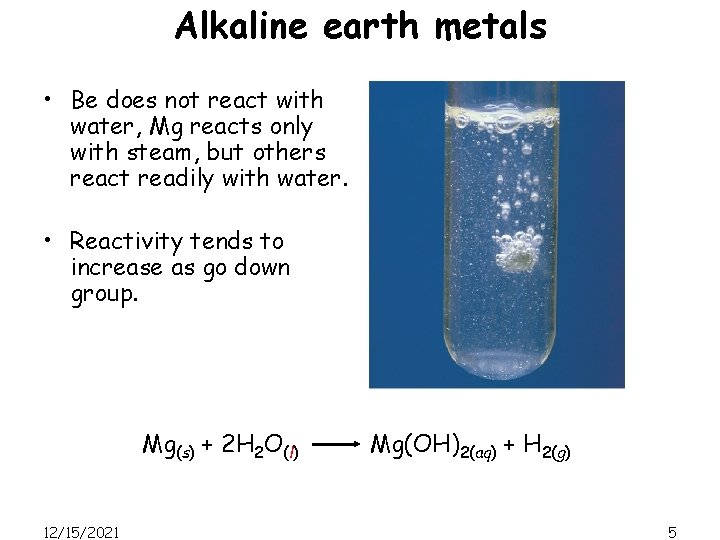
Alkaline earth metals • Be does not react with water, Mg reacts only with steam, but others react readily with water. • Reactivity tends to increase as go down group. Mg(s) + 2 H 2 O(l) 12/15/2021 Mg(OH)2(aq) + H 2(g) 5

Group 3 A elements (ns 2 np 1, n 2) B is a metalloid; boron does not form binary ionic compounds and is unreactive toward O 2 and H 2 O. Al is most abundant metal in Earth’s rest of Group 3 A are metals crust, found as aluminosilicates. 4 Al(s) + 3 O 2(g) 2 Al(s) + 6 HCl(aq) 12/15/2021 2 Al 2 O 3(s) Bauxite is chief ore of Al. 2 Al. Cl 3(s) + 3 H 2(g) 6

Group 3 A elements (ns 2 np 1, n 2) Aluminum forms only tripositive ions. Other 3 A elements form both +3 and +1 states with +1 becoming more stable going down the group 12/15/2021 7

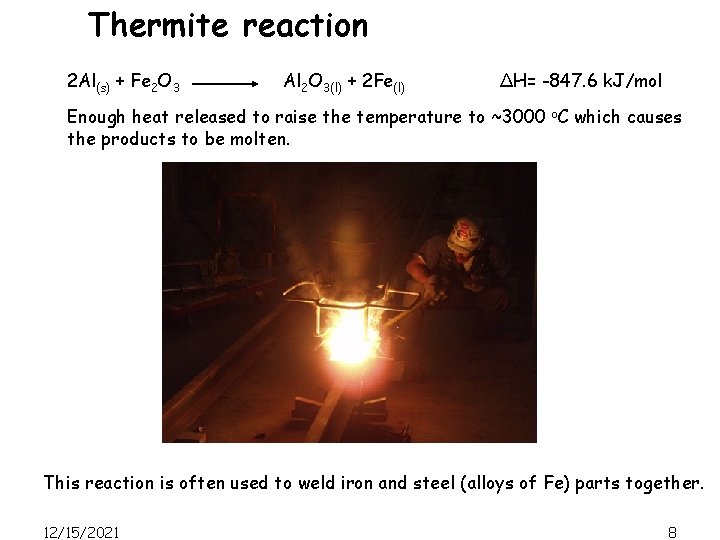
Thermite reaction 2 Al(s) + Fe 2 O 3 Al 2 O 3(l) + 2 Fe(l) ΔH= -847. 6 k. J/mol Enough heat released to raise the temperature to ~3000 o. C which causes the products to be molten. This reaction is often used to weld iron and steel (alloys of Fe) parts together. 12/15/2021 8

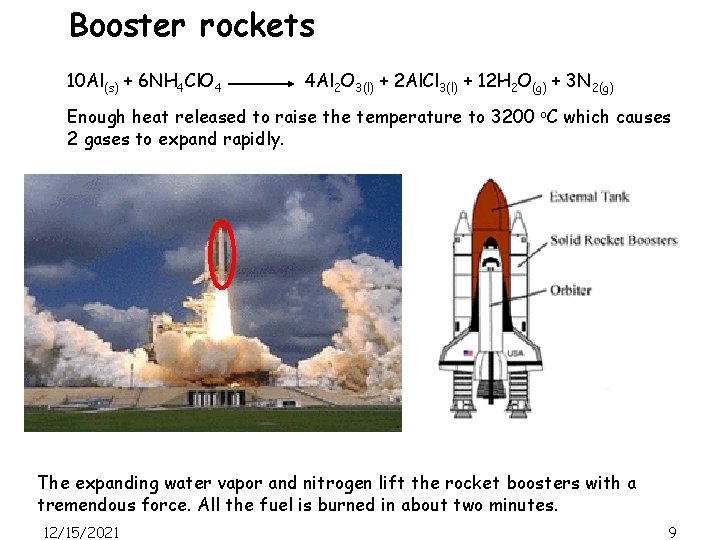
Booster rockets 10 Al(s) + 6 NH 4 Cl. O 4 4 Al 2 O 3(l) + 2 Al. Cl 3(l) + 12 H 2 O(g) + 3 N 2(g) Enough heat released to raise the temperature to 3200 o. C which causes 2 gases to expand rapidly. The expanding water vapor and nitrogen lift the rocket boosters with a tremendous force. All the fuel is burned in about two minutes. 12/15/2021 9

Group 3 A elements (ns 2 np 1, n 2) Aluminum forms only tripositive ions. Other 3 A elements form both +3 and +1 states with +1 becoming more stable going down the group Metallic elements also form many molecular compounds. Al. H 3 resembles Be. H 2 (diagonal relationship, gradual shift from metallic to nonmetallic character from left to right across the periodic table. 12/15/2021 10

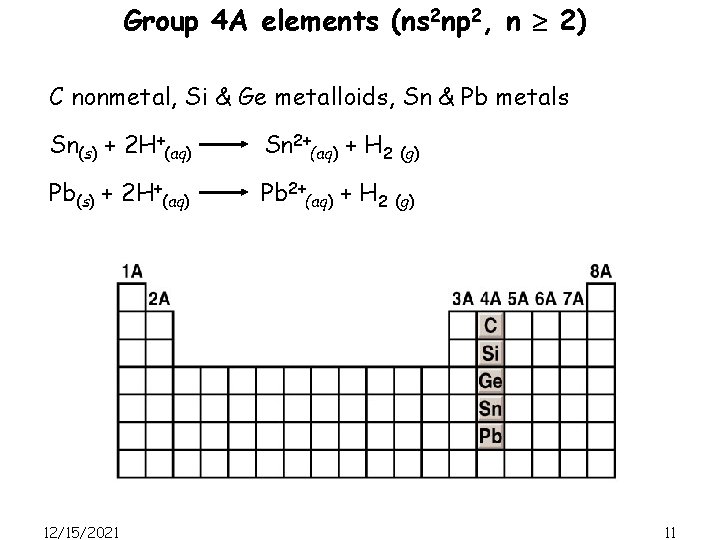
Group 4 A elements (ns 2 np 2, n 2) C nonmetal, Si & Ge metalloids, Sn & Pb metals Sn(s) + 2 H+(aq) Sn 2+(aq) + H 2 (g) Pb(s) + 2 H+(aq) Pb 2+(aq) + H 2 (g) 12/15/2021 11

Group 4 A elements (ns 2 np 2, n 2) 4 A elements form compounds in +2 and +4 oxidation states. For C and Si, +4 oxidation state is more stable. CO 2 more stable than CO Si. O 2 exists; Si. O does not (under normal conditions) +4 is only slightly more stable for Sn; +2 is more stable for Pb 12/15/2021 12

Group 5 A elements (ns 2 np 3, n 2) N & P nonmetals, As & Sb are metalloids, Bi is a metal Elemental N exists as N 2 NO, N 2 O, NO 2, N 2 O 4, and N 2 O 5 N accepts 3 e- to form nitride, N 3 - (most metallic nitrides are ionic compounds) 12/15/2021 13

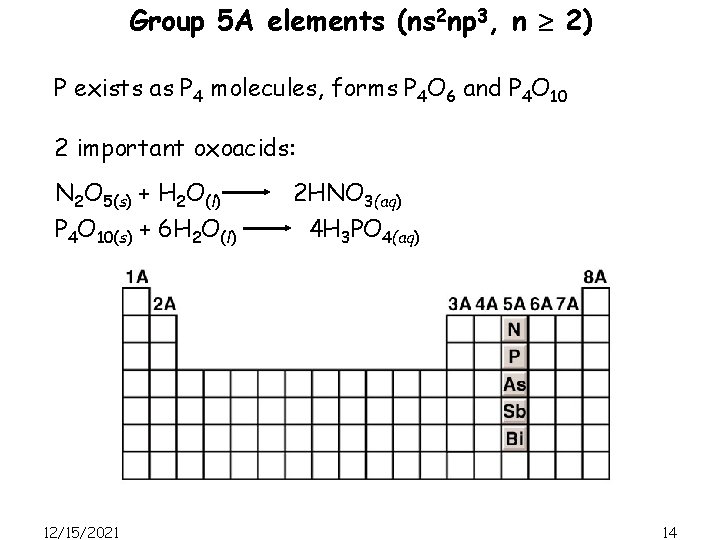
Group 5 A elements (ns 2 np 3, n 2) P exists as P 4 molecules, forms P 4 O 6 and P 4 O 10 2 important oxoacids: N 2 O 5(s) + H 2 O(l) P 4 O 10(s) + 6 H 2 O(l) 12/15/2021 2 HNO 3(aq) 4 H 3 PO 4(aq) 14

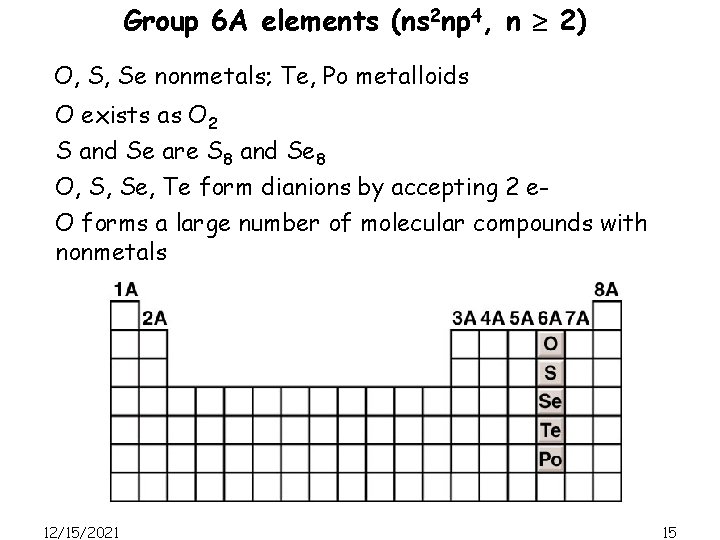
Group 6 A elements (ns 2 np 4, n 2) O, S, Se nonmetals; Te, Po metalloids O exists as O 2 S and Se are S 8 and Se 8 O, S, Se, Te form dianions by accepting 2 e. O forms a large number of molecular compounds with nonmetals 12/15/2021 15

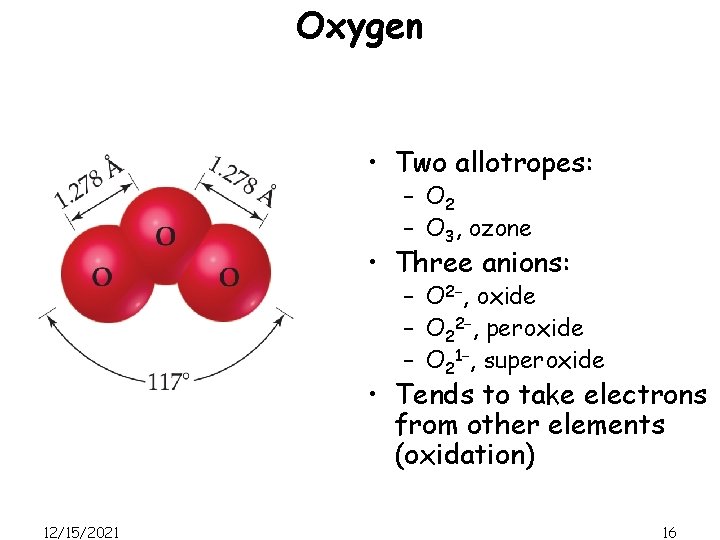
Oxygen • Two allotropes: – O 2 – O 3, ozone • Three anions: – O 2−, oxide – O 22−, peroxide – O 21−, superoxide • Tends to take electrons from other elements (oxidation) 12/15/2021 16

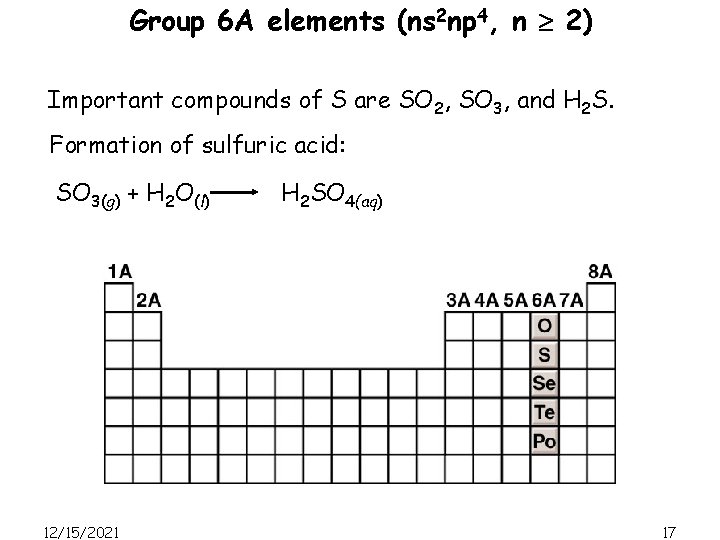
Group 6 A elements (ns 2 np 4, n 2) Important compounds of S are SO 2, SO 3, and H 2 S. Formation of sulfuric acid: SO 3(g) + H 2 O(l) 12/15/2021 H 2 SO 4(aq) 17

Group 7 A elements (ns 2 np 5, n 2) All halogens are nonmetals and exist as X 2 4 HF(aq) + O 2(g) Increasing reactivity 2 F 2(g) + 2 H 2 O(l) 12/15/2021 18

Halogens • Large, negative electron affinities – Therefore, tend to oxidize other elements easily • React directly with metals to form metal halides • Chlorine added to water supplies to serve as disinfectant (HOCl) 12/15/2021 19

Group 7 A elements (ns 2 np 5, n 2) Halogens have high IE and large negative EA X + 1 e. X 2(g) + H 2(g) X -1 (halides) 2 HX(g) Increasing reactivity Form ionic compounds with alkali and alkali earth metals Form molecular compounds among themselves (ICl, Br. F 3) and with nonmetals (NF 3, PCl 5, and SF 6) 12/15/2021 20

Group 7 A elements (ns 2 np 5, n 2) X 2(g) + H 2(g) 2 HX(g) (when X=F, explosive) Increasing reactivity Hydrogen halides dissolve in H 2 O to form hydrohalic acids HF is a weak acid (weak electrolyte) HCl, HBr, HI strong acids (strong electrolytes) 12/15/2021 21

Group 8 A elements (ns 2 np 6, n 2) Completely filled ns and np subshells. Highest ionization energy of all elements. No tendency to accept extra electrons. 12/15/2021 22

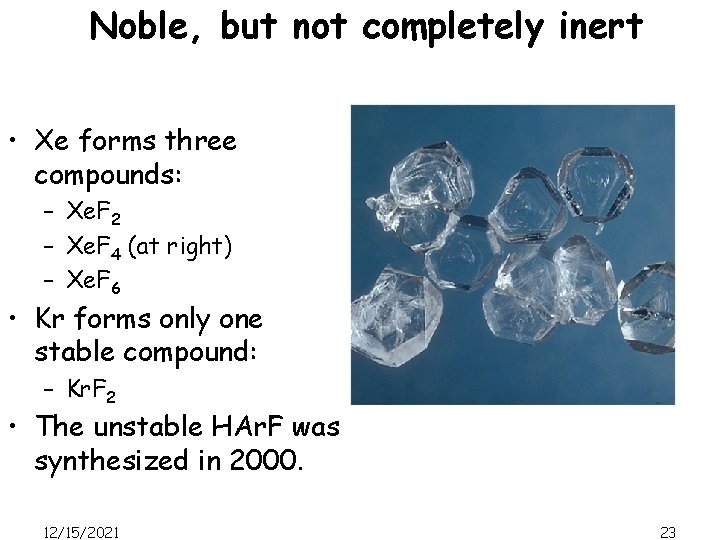
Noble, but not completely inert • Xe forms three compounds: – Xe. F 2 – Xe. F 4 (at right) – Xe. F 6 • Kr forms only one stable compound: – Kr. F 2 • The unstable HAr. F was synthesized in 2000. 12/15/2021 23

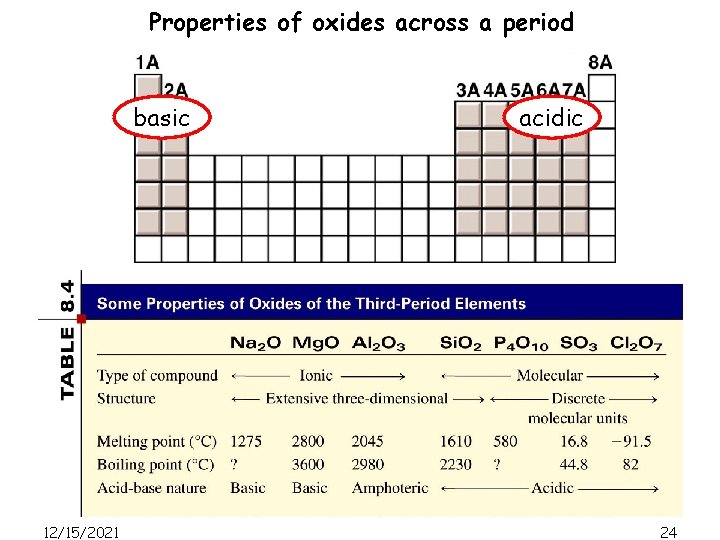
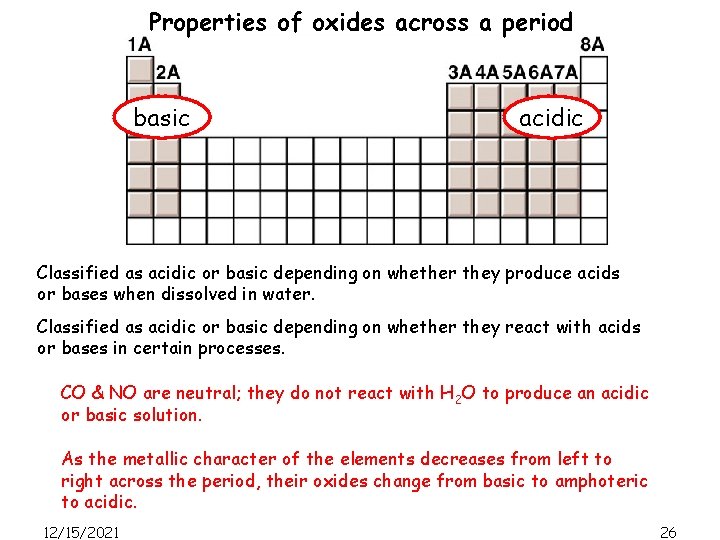
Properties of oxides across a period basic 12/15/2021 acidic 24

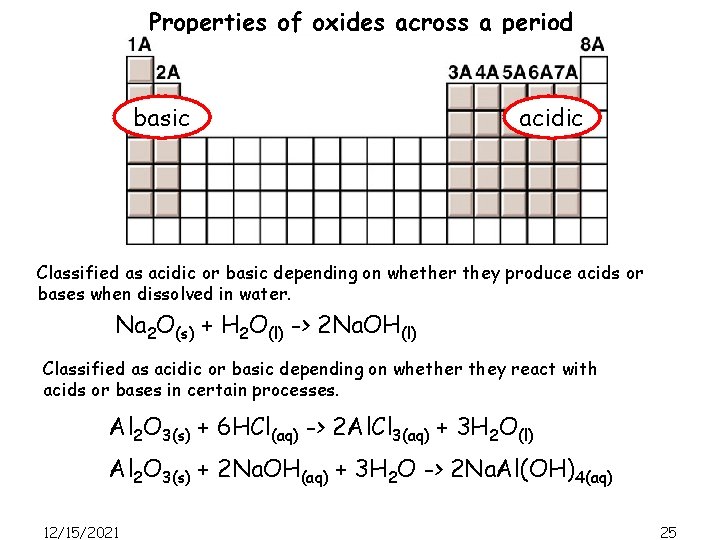
Properties of oxides across a period basic acidic Classified as acidic or basic depending on whether they produce acids or bases when dissolved in water. Na 2 O(s) + H 2 O(l) -> 2 Na. OH(l) Classified as acidic or basic depending on whether they react with acids or bases in certain processes. Al 2 O 3(s) + 6 HCl(aq) -> 2 Al. Cl 3(aq) + 3 H 2 O(l) Al 2 O 3(s) + 2 Na. OH(aq) + 3 H 2 O -> 2 Na. Al(OH)4(aq) 12/15/2021 25

Properties of oxides across a period basic acidic Classified as acidic or basic depending on whether they produce acids or bases when dissolved in water. Classified as acidic or basic depending on whether they react with acids or bases in certain processes. CO & NO are neutral; they do not react with H 2 O to produce an acidic or basic solution. As the metallic character of the elements decreases from left to right across the period, their oxides change from basic to amphoteric to acidic. 12/15/2021 26