Import regulations in major export markets for agro










































- Slides: 60

Import regulations in major export markets for agro products from India on 8 September 2011, IIFT Delhi by Ms. Vidyottama Tripathi Asstt. Director, EIC 1

What are import regulations �Regulations are the mandatory requirement imposed by importing country before its entry �Regulations are divided based on category of products 1. 2. 3. Products intended for human consumption (HC) � Stored at room temperature � Stored under controlled temperature : chilled or frozen Products intended for non-human consumption � Stored at room temperature � Stored under controlled temperature : chilled or frozen Live animals, semen, ova, embryos 2

Import conditions 1 Import in to border only possible if : Ø the product comes from a country or a part of country authorised to export this kind of product Ø the product has been produced in an establishment approved for export Ø Detained , inspected and cleared. 2 Products not allowed for import Redispatch �same means of transport �authorization of competent authorities of origin Ø Destruction : �redispatch impossible �time limit elapsed �decision of the importer �risk for health. 3

Safeguard provisions Ø Serious risk for human or animal health • seizure of the consignment • information to all BIPs (RASFF) • RASFF notification : reinforced checks 10 consignments, 3 months • imports suspended if repeated infringments • specific decisions (lab. analyses) 4

STRATEGIES & INITIATIVES TO MEET IMPORT REGULATIONS 5

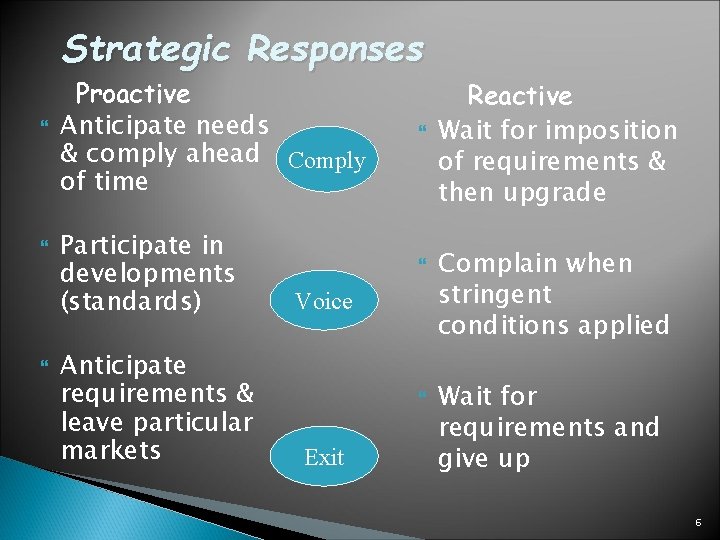
Strategic Responses Proactive Anticipate needs & comply ahead Comply of time Participate in developments (standards) Anticipate requirements & leave particular markets Voice Exit Reactive Wait for imposition of requirements & then upgrade Complain when stringent conditions applied Wait for requirements and give up 6

EXPORT QUALITY CONTROL – A BACKGROUND • Go. I enacted the Export (QC&I) Act, 1963 – an umbrella Act governing quality of exports • Powers of Central Government under the Act Ø Specify standards for export and type of QC & I Ø Notify commodities for compulsory PSI Ø Establish or recognize Agencies for QC & I • EIC set up to advise Government on measures for sound development of exports through QC & I and draw up progs for QC & inspection of commodities • EIC operates certification through 5 EIAs at Mumbai, Kochi, Kolkata, Delhi and Chennai, 28 Sub Offices, Labs and other designated CABs 7

INSPECTION & CERTIFICATION 1 �Product Spectrum ØMandatory export certification – 6 items (Marine, meat , Egg, Dairy, Honey, Poultry Meat, Basmati Riceauthenticity) ØVoluntary - notified (nearly 1000 commodities range from food, footwear, chemicals, st steel, electrical) & non- notified. � Total number of units approved-- B. Rice-06, Black Pepper-8, 8

INSPECTION & CERTIFICATION 2 �Systems of Certification �Systems Approach ØConsignment wise Inspection ØSystems approach ØIn- process Quality Control (IPQC) ØSelf-Certification (SC) ØFood Safety Management Systems based approach �Basis of compliance ØPrimary responsibility on processor – develop & maintain systems ØEIC to approve & ensure compliance 9

INSPECTION & CERTIFICATION -3 � Approval/renewal of units through a 2 -level process (Conditional & final) � Compliance ensured through 3 -tier surveillance system - Risk based monitoring � Implementing HACCP-based systems; aligned with Codex/EC requirements � Traceability & Primary production issues – addressing farms controls, upgrading landing centres, fishing boats, residue monitoring & control, awareness programmes � Testing as/ latest technology & systems reqts (Pesticide residues, antibiotic residues, heavy metals, microbiological parameters, water) � Complaint addressing system 10

RECOGNITIONS-1 � EC - Designated CA for marine products & basmati rice; dialogue on for dairy, egg, poultry meat, honey � USA (USFDA) - recognized for Black Pepper – no detention if accompanied by EIC certificate; started dialogue for poultry � Singapore – MRA in area of food & agri, electrical & electronics, drugs, telecommunication � Australia (AQIS) - recognized for marine products – seeking for dairy, spices, honey, etc � Sri Lanka (SLSI) - recognized for >100 regulated prods (food, cement, engineering/electrical, etc) 11

RECOGNITIONS -2 � Italy – fish & fishery products � Japan – recognized EIC certification for poultry products wef 13 October 2005 � Turkey – recognized EIA health certificates for all food items, stainless steel utensils & packaging material for foods � S. Korea (KFDA)- recognised for food/ agriculture prods � China – Agreement on Iron ore signed on 21 November 2006 � Nepal – all agriculture & food products � Russia – Recognized for Marine Products 12

EC regulation for non animal origin foods �A general obligation of operator to monitor food safety of product & process (Article 3 of reg (EC) no. 852/2004 � General Hygienic provision of primary production (Article 4. 1 of part A of Annex I to Reg (EC) no. 852/2004 � Detailed reqm after primary production (Article 4. 2 of and Annex II to Reg (EC) no. 854/2004 � Microbiological criteria as per article 4. 3 of 852/2004 and commission regulation 2073/2005 � Procedures to be based on HACCP Principles (Article 5 of reg 852/2004 � Registration of establishment (Article 6 of regulation (EC 852/2004 13

EC regulation for non animal origin foods �Health Requirements ü Contaminants eg in basmati rice- 5&10 ug/kg ü MRLs for pesticide residue (in basmati ricecarbendazim, benomyl, cyfluthrin, thiophanate-methyl ü Use of Food Additives ü Food Irradiation �Products specific requirements ü Non GMO certificate 14

LATEST Developments � Vide EC Regulation NO. 799/2011 of 9 August 2011 ü Curry leaves – due to pesticides residue problem 10% physical checks ü Chillies & spices- due to aflatoxin problem 50% physical checks. For Sudan dyes- 10% ü Ground nuts- due to aflatoxin problem 20% physical checks ü Fresh okra- due to pesticides residue problem 10% physical checks 15

Regulation (EC) No 396/2005 of 23 February 2005 on MRLs of pesticides in or on food and feed of plant and animal origin Foodstuffs concerned � � � It listed MRLs for 1270 pesticides for products intended for human or animal consumption listed in Annex I. not subject to the set limits if they are intended for sowing or planting or export outside the European Union. Default limit and specific limits MRL in foodstuffs- 0. 01 mg/kg. This general limit is applicable ‘by default’, i. e. in all cases where an MRL has not been specifically set for a product or product type. specific MRLs listed in Annex II are higher than the default limit. 16

Regulation (EC) No 396/2005 of 23 February 2005 on MRLs of pesticides in or on food and feed of plant and animal origin � In some cases, provisional MRLs -listed in Annex III, in particular be set in the following cases: ü ü for honey and herbal infusions; in certain exceptional circumstances of contamination by plant protection products; for national MRLs which have not yet been harmonised; where new products are listed in Annex I and a Member State requests it, in order to have enough time for a comprehensive scientific assessment and provided that no risk to the consumer has been detected. � Products which do not comply with the fixed limits may not be diluted except in the case of certain processed and/or composite products listed by the Commission (Annex VI). 17

To access the database, http: //ec. europa. eu/sanco_pest icides/public/index. cfm 18

19

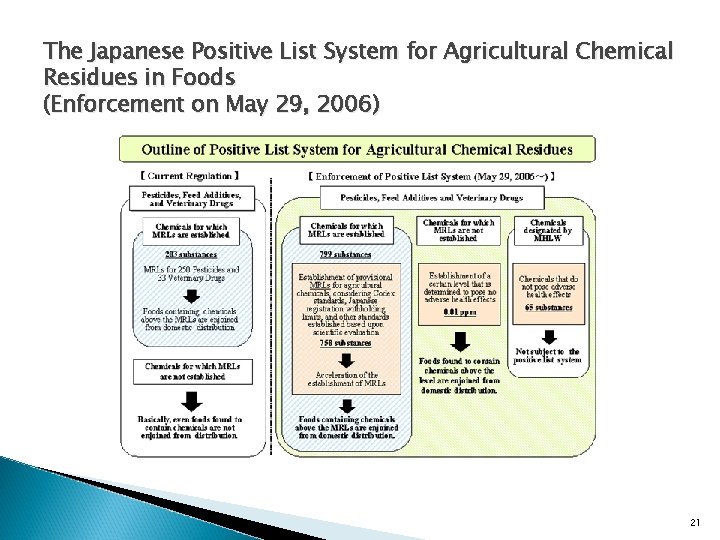
The Japanese Positive List System for Agricultural Chemical Residues in Foods (Enforcement on May 29, 2006) � � � Maximum Residue Limits (MRLs) of Agricultural Chemicals in Foods in which any agricultural chemical residues (including pesticides, feed additives, and veterinary drugs) are found in excess of the level shall not be produced, imported, processed, used, cooked, or stored for sale, or sold, provided, (Summary of Article 11, Paragraph 3 of the Food Sanitation Law) Uniform Limit: 0. 01 ppm The level determined by the Minister of Health, Labour and Welfare (MHLW Notification No. 497, 2005) List: Total – 65 substances having no potential to cause damage to human health and specified so by the Minister of Health, Labour and Welfare. (MHLW Notification No. 498, 2005) 20

The Japanese Positive List System for Agricultural Chemical Residues in Foods (Enforcement on May 29, 2006) 21

http: //www. ffcr. or. jp/zaidan/F FCRHOME. nsf/pages/MRLs-p 22

http: //www. apvma. gov. au/residues/st andard. php#background 23

Gulf Countries �Maximum Limits of Pesticide Residue in Agricultural and Food Products Part 1 — UAEs GSO 382/1994 and Maximum Limits of Pesticide Residue in Agricultural and Food Products Part 2 — UAEs GSO 383/1994. Countries included in the GCC are: Saudi Arabia, United Arab Emirates, Kuwait, Bahrain, Oman and Qatar. If an MRL is ‘not set’, generally, the GCC will use the Codex MRL. 24

US Import regulation 1 � All foods imported into the U. S. must meet the same requirements as those produced domestically. � In the U. S. , food safety is a shared responsibility with several departments of the United States government sharing jurisdiction over ensuring the safety of the American food supply.

US Import regulation 2 � 1. 2. 3. 4. 5. Agencies involved include: The Food and Drug Administration as FDA - regulates both domestic and imported foods, except meat, poultry and processed eggs, and has primary responsibility for enforcing food safety laws including food import and export regulations. The Centers for Disease Control and Prevention as CDCP - works closely with state and local public health epidemiologists and laboratories to identify Illnesses and clusters of illnesses that may be foodborne. The U. S. Department of Agriculture as USDA -has several agencies that carry out a wide range of programs that may play a role in assuring food safety by establishing the safety of imported fruits and vegetables. The U. S. Environmental Protection Agency as EPA - regulates pesticides and assures drinking water meets standards for health. The U. S. Customs Service - serves as the point of entry for products imported into the U. S.

US Import regulation 3 APHIS Import Authorization System USDA through the Animal Plant Health and Inspection Service (APHIS) requires � permits for certain fresh fruits and vegetables that are imported from any foreign country. � Entry requirements can be obtained from the Import Authorization System � available on USDA’s website http: //www. aphis. usda. gov/oa/new/at. html. .

The FAO/WHO International Conference on Nutrition, 1992 Recognized that: � access to nutritionally adequate and safe food is a right of each individual, � Food regulations… should fully take international standards of the Codex Alimentarius

Codex Alimentarius is: Latin term for “ Food Law” or “Food Code”

Codex Alimentarius Commission �FAO in 1961 and WHO 1963 passed Resolution to establish the Codex Alimentarius Commission, �Responsible for Joint FAO/WHO Food Standard Program.

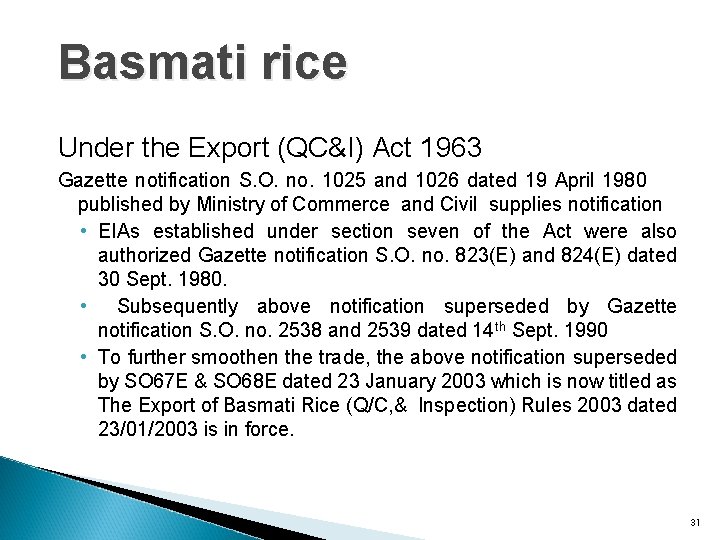
Basmati rice Under the Export (QC&I) Act 1963 Gazette notification S. O. no. 1025 and 1026 dated 19 April 1980 published by Ministry of Commerce and Civil supplies notification • EIAs established under section seven of the Act were also authorized Gazette notification S. O. no. 823(E) and 824(E) dated 30 Sept. 1980. • Subsequently above notification superseded by Gazette notification S. O. no. 2538 and 2539 dated 14 th Sept. 1990 • To further smoothen the trade, the above notification superseded by SO 67 E & SO 68 E dated 23 January 2003 which is now titled as The Export of Basmati Rice (Q/C, & Inspection) Rules 2003 dated 23/01/2003 is in force. 31

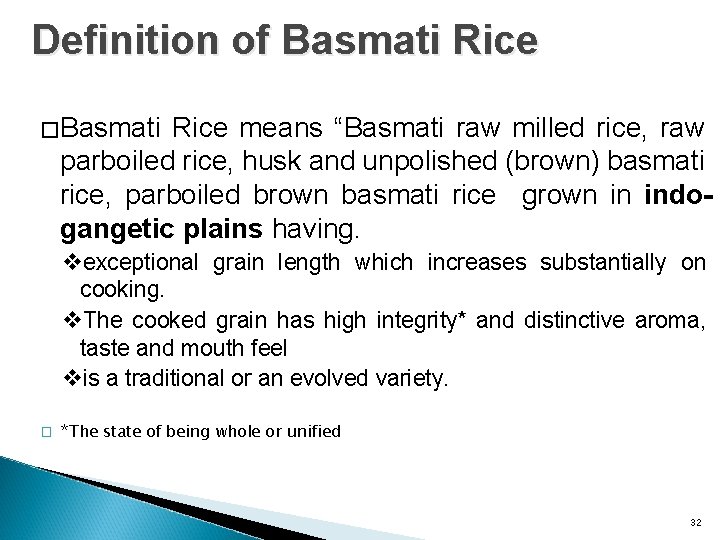
Definition of Basmati Rice �Basmati Rice means “Basmati raw milled rice, raw parboiled rice, husk and unpolished (brown) basmati rice, parboiled brown basmati rice grown in indogangetic plains having. vexceptional grain length which increases substantially on cooking. v. The cooked grain has high integrity* and distinctive aroma, taste and mouth feel vis a traditional or an evolved variety. � *The state of being whole or unified 32

Definition …. . Contd. • “Brown Rice” means where husk is removed leaving bran intact on the grain. • “Brown Rice Parboiled” means rice grain obtained when paddy is parboiled and husked or shelled. • “Raw Milled Rice” means rice grains obtained after the process of shelling and milling of paddy resulting in removal of bran from brown rice. • “Raw Parboiled Rice” means rice grains obtained when paddy is parboiled, husked and milled resulting in removal of bran from brown 33

Certification system The inspection of Basmati Rice shall be carried out to ascertain that the same conforms to the standard specification recognized under section 6 of the Act. �Consignment Wise Inspection (CWI) system �Processing/ milling unit approval under In Process Quality Control system (IPQC). 34

Consignment Wise Inspection (CWI) System � Ø Ø Ø Ø An exporter intend to export shall prepare the consignment of Basmati Rice in a manner that the same can form any of three grade i. e. Special, A or B prescribed in the notification and packed in new gunny bags with the following information. Name and address of the exporter Name of the product Variety & grade Lot no. Gross weight and net weight. Product of India Shipping mark 35

CWI…… Contd. Notice of Intimation: The exporter shall submit the following at the nearest office of EIA § § Intimation for inspection in duplicate Invoice copy Purchase order Inspection fee @ 0. 4% of fob Place of Inspection § The inspection shall be carried out at the premises where the consignment is offered. § Exporter shall provide the adequate facilities for inspection. 36

CWI…… Contd. SAMPLING PROCEDURE Make homogeneous composite sample weighing about 3. 5 Kgs. � divide the composite sample into 5 final samples 2 weighing 1 kg and rest 3 weighing 500 gms. � pack the final sample in a cloth bag with identification mark, which shall contain intimation no. , date of inspection, lot no. , product, variety, grade, name designation and signature of the inspecting officer. � seal the samples with lead seal using pliers. � hand over one sealed sample to the exporter under proper acknowledgement � bring back the other sealed samples and hand over to the controlling officer. � prepare a Field Inspection Report (FIR) in respect of each consignment on completion of inspection. 37

CWI…… Contd. Certification of Inspection § § § If sample is found conforming to the specification, certificate of inspection with 45 days validity from the date of completion of inspection If sample does not conform to the specification, EIA shall issue a rejection letter stating reason of rejection. extension of 15 days validity 38

Procedure for approval- IPQC system The unit having all requisite facilities may submit an application in a prescribed proforma to the nearest EIA office along with following documents. �Application �Layout plan of the unit. �Legal identity of the unit. �List of machineries and equipments. �List of qualified technical staff. 39

Procedure for approval § § § Application found complete in all respect, EIA shall depute an officer to conduct survey of the unit. On the basis of survey report if the unit is found to be meeting the norms, Inter Departmental panel (IDP )shall be arranged. IDP shall be constituted from the following departments. • • • § Agricultural Products Export Development Authority. (APEDA) Indian Agriculture Research Institute (IARI) Food Corporation of India (FCI) Joint Commissioner (SRR), Ministry of Consumer Affairs, GOI Deputy Director, EIA (Convener) Minimum quorum -3 40

Procedure for approval ◦ If the unit is recommended by IDP and its recommendation is accepted, the following actions are taken �Allot an approval number which shall be unique for each unit- BR-04 -001 �Issue a letter of approval to the unit with a copy to EIC. ◦ EIC shall issue Certificate of Approval to all approved unit in prescribed format. ◦ Validity of the approval granted to the unit for a period of 2 years. 41

Surveillance of the approved unit 3 tier surveillance system I Monitoring visit : • Frequency of Monitoring visit shall be carried out once in three months. • The monitoring officer at the level of field officer verify the following ØRaw material, process and product control, ØStorage and transportation control ØHygiene and sanitation control ØVerification of laboratory equipments and test records ØDocuments and records. q 42

Surveillance of the Approved Unit �Monitoring officer shall also draw one sample from consignment available for export and send the same to EIA laboratory for testing as per notified std. �In case of any failure of sample tested, all the subsequent consignments to be checked till such time three consecutive consignments are found conforming to notified std. 43

Surveillance of the Approved Unit Supervisory visit § Frequency- once in 6 months. §Performance of laboratory equipments and testing § Documents as per prescribed format Corporate audit §Frequency -once in a year §Examination of records of processor maintained by the agency like visit report, laboratory report etc. §Visit by audit team at least 10% of the units. §Audit team comprises of at least two officers from EIC/EIA. 44

Renewal of approval Øapply at least 60 days before expiry of earlier approval. ØApproval may be renewed on the basis of assessment conducted by IDP ØApproval of renewal is valid for a period of 2 years. 45

Certification The IPQC unit shall issue certificate of inspection themselves ØBefore issuing certificate of inspection, processor shall ensure that the product conforms to the specification recognized by GOI/EIC ØShall be issued by authorized official not below the rank of head of quality control department. ØEvery certificate issued shall bear a distinct serial number which never be repeated and date of issue. ØThe certificate shall be neatly typed. If any correction, the same must be counter signed by the certifying officer. ØValidity of certificate is 45 days from the date of issue. 46

Norms of approval of IPQC unit of Basmati Rice • Surrounding and constructions. • Raw material control • Process control • General sanitation with in the plant • Product control • Pest control • Equipment and meteorological control • Personnel hygiene • Packing • Transportation 47

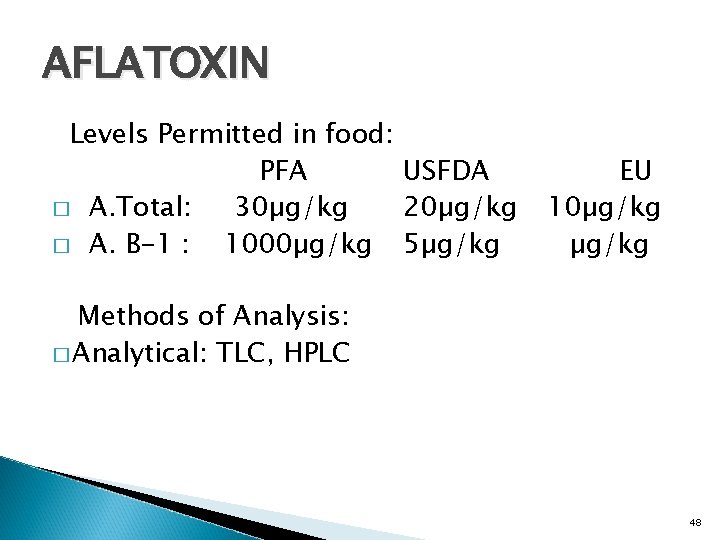
AFLATOXIN Levels Permitted in food: PFA USFDA � A. Total: 30µg/kg 20µg/kg � A. B-1 : 1000µg/kg 5µg/kg EU 10µg/kg Methods of Analysis: � Analytical: TLC, HPLC 48

Aflatoxin Control by EIC/EIA � Consignment wise inspection � Sample from each consignment drawn and tested � IPQC system : One sample in a month drawn and tested from IPQC unit 49

REGULATIONS (EC 1881/2006) setting maximum levels for certain contaminants in foodstuffs regards aflatoxins followed � Sampling method are followed as per (EC) Regulation No. 401/2006 for Alfatoxin 50

Recent Development Regarding Basmati Rice Export To U. S. A • Ministry of agriculture has received complaints regarding presence of khapra beetle in the rice being exported to U. S. A. A series of meeting pertaining to same, have taken place between EIA/EIC, Agricultural and processed food products export development authority(APEDA), Directorate of plant protection, quarintine and warehouse(DPPQS), Ministry of agriculture. • As a results of this all establishment intending ot export ot USA need prior approval • 51

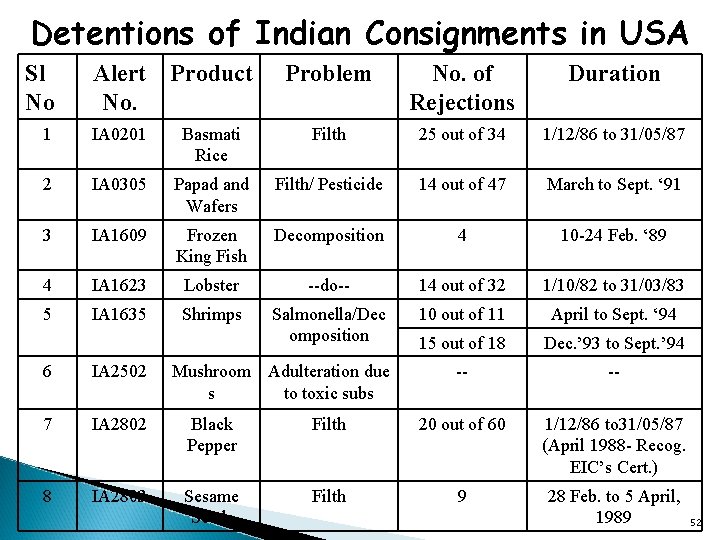
Detentions of Indian Consignments in USA Sl No Alert Product No. Problem No. of Rejections Duration 1 IA 0201 Basmati Rice Filth 25 out of 34 1/12/86 to 31/05/87 2 IA 0305 Papad and Wafers Filth/ Pesticide 14 out of 47 March to Sept. ‘ 91 3 IA 1609 Frozen King Fish Decomposition 4 10 -24 Feb. ‘ 89 4 IA 1623 Lobster --do-- 14 out of 32 1/10/82 to 31/03/83 5 IA 1635 Shrimps Salmonella/Dec omposition 10 out of 11 April to Sept. ‘ 94 15 out of 18 Dec. ’ 93 to Sept. ’ 94 -- -- 6 IA 2502 Mushroom Adulteration due s to toxic subs 7 IA 2802 Black Pepper Filth 20 out of 60 1/12/86 to 31/05/87 (April 1988 - Recog. EIC’s Cert. ) 8 IA 2803 Sesame Seeds Filth 9 28 Feb. to 5 April, 1989 52

Detentions without physical examination imposed � Reason for Alert: From December 1, 1986, through May 31, 1987, 25 of 34 shipments of Basmati rice from India (73%) were detained for filth (rodent, insect, bird, extraneous materials). Of the 25 detentions, 12 were for rodent filth or rodent and insect filth, 12 were for insect filth and extraneous materials, and 1 for insect and bird filth. . 53

Black Pepper -Reason for Alert � � � From December 1, 1986, through May 31, 1987, 20 out of 60 shipments of black pepper (peppercorns) that were sampled, or 33 percent, were detained for filth. The 20 detentions represented 11 different shippers. These findings resulted in black pepper from India being placed under automatic detention in July 1987. In April 1988, a certification program introduced Under this program the EIC is sample and test each lot of black pepper exported to the United states; only permit those lots that comply with FDA's requirements for Salmonella, filth, mold and foreign matter to be exported to the United States; Because of the apparently successful certification program initiated by the EIC Government of India, detention without physical examination of Indian black pepper shipments will not be invoked when such shipments are accompanied by certificates. � 54

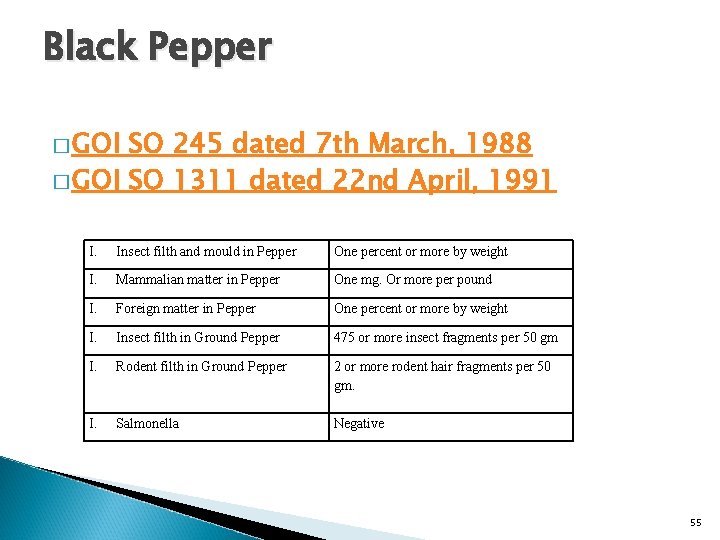
Black Pepper � GOI SO 245 dated 7 th March, 1988 � GOI SO 1311 dated 22 nd April, 1991 I. Insect filth and mould in Pepper One percent or more by weight I. Mammalian matter in Pepper One mg. Or more per pound I. Foreign matter in Pepper One percent or more by weight I. Insect filth in Ground Pepper 475 or more insect fragments per 50 gm I. Rodent filth in Ground Pepper 2 or more rodent hair fragments per 50 gm. I. Salmonella Negative 55

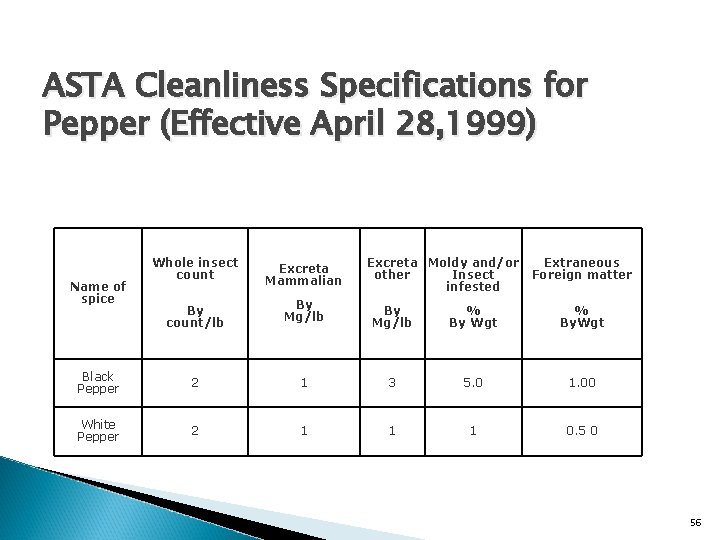
ASTA Cleanliness Specifications for Pepper (Effective April 28, 1999) Whole insect count Excreta Mammalian By count/lb By Mg/lb % By Wgt % By. Wgt Black Pepper 2 1 3 5. 0 1. 00 White Pepper 2 1 1 1 0. 5 0 Name of spice Excreta Moldy and/or other Insect infested Extraneous Foreign matter 56

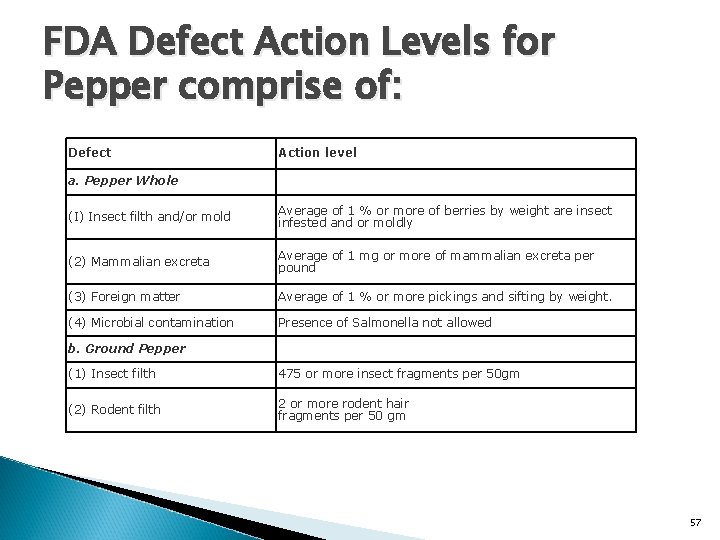
FDA Defect Action Levels for Pepper comprise of: Defect Action level a. Pepper Whole (I) Insect filth and/or mold Average of 1 % or more of berries by weight are insect infested and or moldly (2) Mammalian excreta Average of 1 mg or more of mammalian excreta per pound (3) Foreign matter Average of 1 % or more pickings and sifting by weight. (4) Microbial contamination Presence of Salmonella not allowed b. Ground Pepper (1) Insect filth 475 or more insect fragments per 50 gm (2) Rodent filth 2 or more rodent hair fragments per 50 gm 57

Black Pepper-Pesticide Residue limits Pesticide Tolerance Limit Dieldrin 0. 05 ppm BHC 0. 05 ppm Chlordane 0. 1 ppm Heptachlor 0. 0 I ppm Malathion 0. 1 ppm Parathion 0. 3 ppm Aflatoxin BI 2 ppb (Maximum) B I + B 2 + G I + G 2 4 ppb (Maximum ) 58

Black Pepper � All consignment to be detained (including whole peppercorns, ground, crushed, etc) from India not accompanied by a certificate from the Indian EIC certificates should contain the following information: � � � Lot identification number; Number and size of containers in the lot; Analytical methodology used to determine levels of adulterants/ contaminants; Analytical results of tests for Salmonella, filth, mold and foreign matter; Date of certificate; and Name and stamp or seal of authorizing official. 59

Thank you 60