Implementing screening for acute HIV infection in STD













- Slides: 16

Implementing screening for acute HIV infection in STD clinics already using rapid HIV antibody testing, New York City, 2007 Kathleen D. Gallagher, MPH 1 Pragna Patel, MD MPH 2, Alexis Kowalski, MPH 1, Ellen Klinger, MPH 1, Kathy Gombel 3, Tim Sullivan 3, Monica Parker 3, Ph. D, Susan Blank, MD MPH 1, 2 1 New York City Department of Health & Mental Hygiene (NYC DOHMH) 2 Centers for Disease Control & Prevention (CDC) 3 New York State Department of Health (NYS DOH)

Acute HIV Infection (AHI) n n Highly infectious and often symptoms not recognized Not detectable by routine antibody tests, requires direct detection of HIV virus Important to diagnose as it poses high impact opportunity to interrupt the spread of disease Screening all STD clinic-based testers could have large impact on decreasing new HIV infections

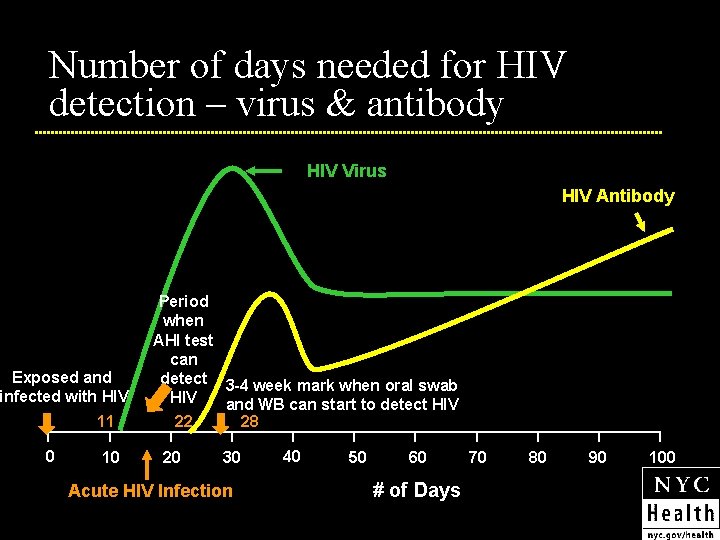
Number of days needed for HIV detection – virus & antibody HIV Virus HIV Antibody Exposed and infected with HIV 11 0 10 Period when AHI test can detect 3 -4 week mark when oral swab HIV and WB can start to detect HIV 22 28 20 30 Acute HIV Infection 40 50 60 # of Days 70 80 90 100

New York City Dept. of Health STD Clinics n n 10 clinics - 136, 109 visits in 2006 All patients offered HIV antibody testing Confirmatory blood routinely collected for all testers; discarded if rapid test negative Individual tests for HIV virus available on a case-by-case basis as part of routine care

Project Objective n Develop system to screen and identify AHI: n n n Acceptable to patients Clinically useful Operationally feasible

Methods n n n National CDC-sponsored feasibility study Convenience sample at 3 of our STD clinics Eligibility Requirements: n n Confidential HIV antibody tester >18 yrs of age Signed research consent Routinely collected whole blood specimens tested for HIV RNA via nucleic acid amplification testing (NAAT) at Wadsworth Lab

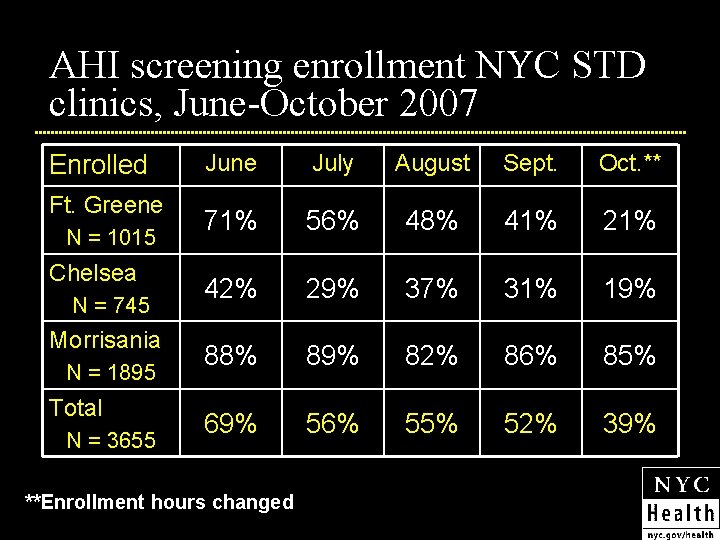
AHI screening enrollment NYC STD clinics, June-October 2007 Enrolled Ft. Greene N = 1015 Chelsea N = 745 Morrisania N = 1895 Total N = 3655 June July August Sept. Oct. ** 71% 56% 48% 41% 21% 42% 29% 37% 31% 19% 88% 89% 82% 86% 85% 69% 56% 55% 52% 39% **Enrollment hours changed

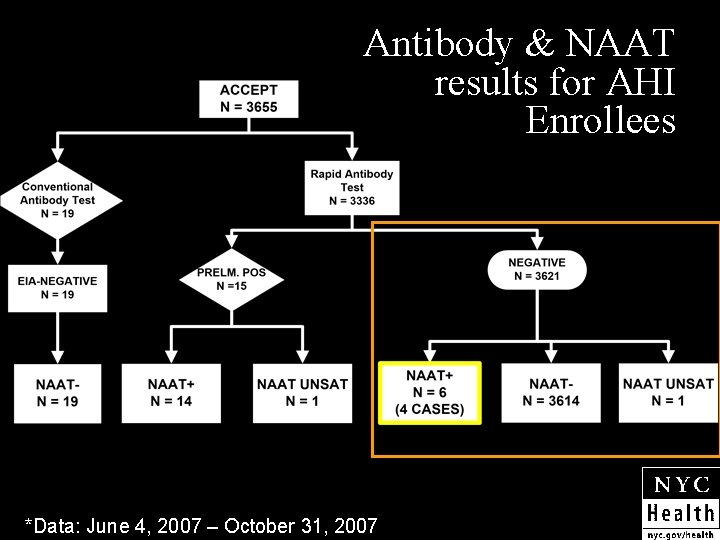
Antibody & NAAT results for AHI Enrollees *Data: June 4, 2007 – October 31, 2007

Descriptive Epi n Difference between those who accepted AHI and those who refused AHI were examined: n n Gender Race/ethnicity Sexual risk factor Age group Significant p<0. 001

Operational Issues n n n Shipping requirements limited enrollment hours Consenting process added additional time to clinic visit NAAT results take 5 -7 days to return and introduced the need for participants to retrieve results

Summary n n Due to the limitations of HIV antibody testing we are missing acutely infected individuals Pooled HIV NAAT is an economical way to screen rapid antibody negative specimens for HIV virus Additional consent form posed a barrier in NYC With opt-in testing more worried well than truly high risk patients accepted additional HIV NAAT

Why continue HIV NAAT? n n Routinizing pooled HIV NAAT screening as part of standard consent would allow testing of all high risk individuals Identified 4 cases of acute HIV out of 3621 pooled rapid HIV negative samples (0. 11%) NYC DOHMH clinics performed 53, 169 HIV antibody tests in 2006 Adding HIV NAAT could help us identify ~50 acutely infected individuals each year

Lessons Learned n n n Simplify consent process Decrease test turn around time - time between testing and receiving results Reduce the labor intensity of specimen processing

Next Steps n n Work to incorporate new language into current NYS HIV consent form to allow for routine HIV NAAT Streamline specimen packaging for local pick-up and testing Collaborate with local Public Health Lab (PHL) to create infrastructure for HIV NAAT Develop electronic mechanisms for data exchange with local PHL

Acknowledgements n n Pragna Patel, MD MPH NYC DOHMH n n Chelsea, Ft. Greene, Chelsea clinic staff Public Health Laboratory Alexis Kowalski (Project Coordinator) NYS DOH Wadsworth Center n n Tim Sullivan Kathy Gombel

Contact Information n Kathleen Gallagher n n kgallagh@health. nyc. gov (212) 788 -6614