Implementing NAT Universal Parasitic Screening for Blood Donors
Implementing NAT& Universal Parasitic Screening for Blood Donors Prof. Dr. Ayesha Junaid MBBS, MCPS, FCPS, MCCQE Program Director Haematology Incharge Blood transfusion Services Shifa International Hospital Islamabad. Pakistan
Implementing NAT& Universal Parasitic Screening for Blood Donors My Talk Today: o Transfusion Therapy o Donor Selection Criteria & Infectious Screening o Transfusion in Pakistan o Our experience o Future vision
Implementing NAT& Universal Parasitic Screening for Blood Donors. Transfusion Therapy: o Life Saving Intervention o >100 million donations collected globally/year o 180 countries ( 7 billion/ 98. 3%) of the global population: 112. 5 million blood donations were made Global Status Report on Blood Safety and Availability, 2016 WHO, Global Database on Blood Safety (GDBS).
SAFE TRANSFUSION IS CRUCIAL IN PATIENT CARE
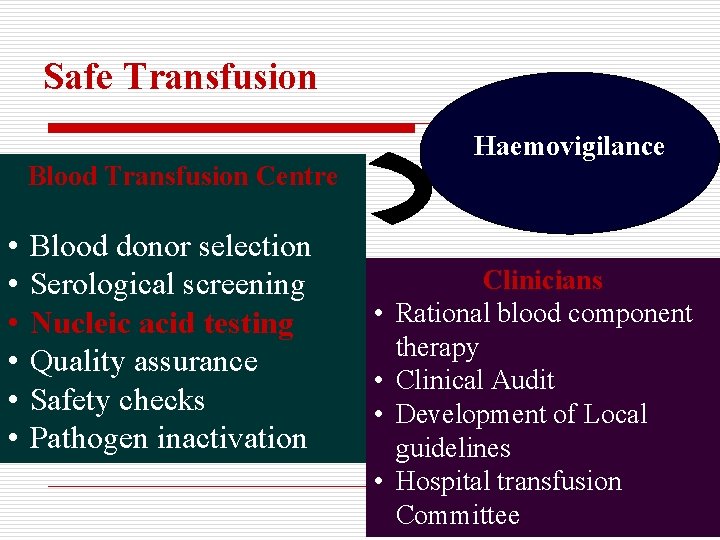
Safe Transfusion Haemovigilance Blood Transfusion Centre • • • Blood donor selection Serological screening Nucleic acid testing Quality assurance Safety checks Pathogen inactivation • • Clinicians Rational blood component therapy Clinical Audit Development of Local guidelines Hospital transfusion Committee

Implementing NAT& Universal Parasitic Screening for Blood Donors Timely Access to Safe Blood Uncertain The safety and availability of blood components requires o Voluntary Non-remunerated donors o Quality-assured Screening Protocols o Safe and rational clinical use of blood.
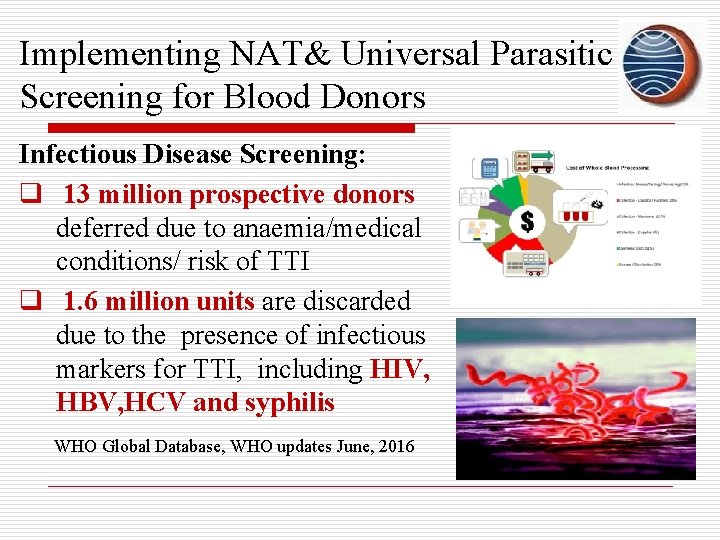
Implementing NAT& Universal Parasitic Screening for Blood Donors Infectious Disease Screening: q 13 million prospective donors deferred due to anaemia/medical conditions/ risk of TTI q 1. 6 million units are discarded due to the presence of infectious markers for TTI, including HIV, HBV, HCV and syphilis WHO Global Database, WHO updates June, 2016

Implementing NAT& Universal Parasitic Screening for Blood Donors q Prior to 1985, only two infectious disease screening assays were performed on donated blood o MP NAT was added to routine serological screening in 1999. o Specific testing protocols vary by country and sometimes by region
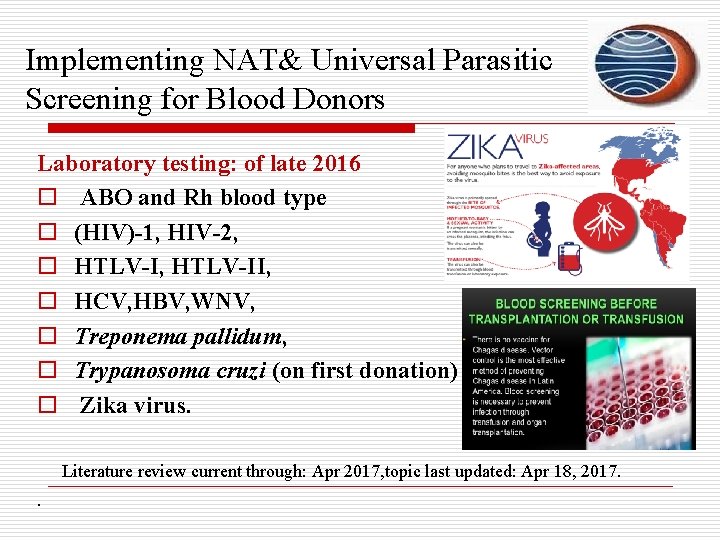
Implementing NAT& Universal Parasitic Screening for Blood Donors Laboratory testing: of late 2016 o ABO and Rh blood type o (HIV)-1, HIV-2, o HTLV-I, HTLV-II, o HCV, HBV, WNV, o Treponema pallidum, o Trypanosoma cruzi (on first donation) o Zika virus. Literature review current through: Apr 2017, topic last updated: Apr 18, 2017. .
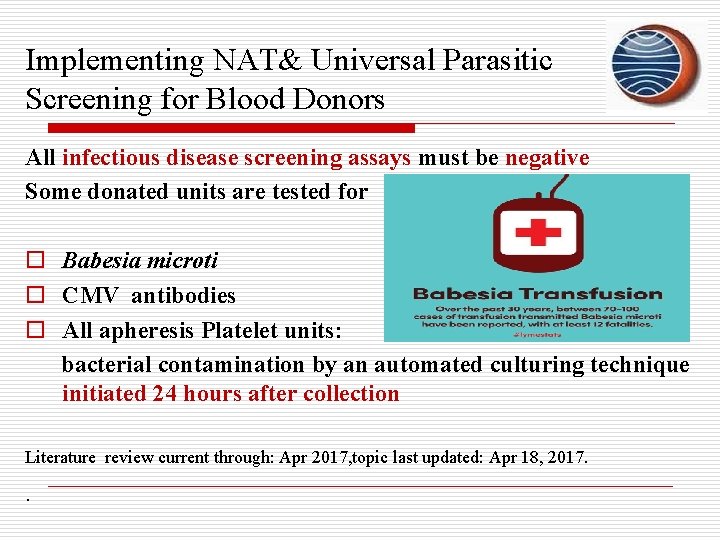
Implementing NAT& Universal Parasitic Screening for Blood Donors All infectious disease screening assays must be negative Some donated units are tested for o Babesia microti o CMV antibodies o All apheresis Platelet units: bacterial contamination by an automated culturing technique initiated 24 hours after collection Literature review current through: Apr 2017, topic last updated: Apr 18, 2017. .

Implementing NAT& Universal Parasitic Screening for Blood Donors Window Period: o During which a donor can be infected, but have negative serological screening tests Window phase for current antibody tests o 22 days : HIV o 59 days : HBV o 70 days : HCV 11 days 34 days 23 days

Nucleic Acid Testing (NAT) Reduction in Serological Window Period NAT positive Immune reaction Serological positive Virus Infection Earlier detection Time

Implementing NAT& Universal Parasitic Screening for Blood Donors o NAT was adopted voluntarily by plasma fractionation companies in Europe in mid-1990 s. o In the late 1990 s in US. and many countries across Europe. o Initially, NAT became mandatory in the developed countries only for HCV & HIV-1 screening, but later started NAT for HBV especially in countries with a high prevalence. Barbara JA. NAT: perspectives for cellular components. Biologicals 1999; 27: 333 -6 Roth WK, Weber M, Seifried E. Feasibility and efficacy of routine PCR screening of blood donations for HCV, HBV, & HIV-1 in a blood-bank setting. Lancet 1999; 353: 359 -63.

Implementing NAT& Universal Parasitic Screening for Blood Donors o Pakistan's annual requirement: 1. 5 million units o Blood transfusion services: Mostly hospital-based. o 1820 blood banks in the country. o > 90% of total blood transfused is replacement donation. www. sbtp. gov. pk 2016 Report. WHO EMRO Blood safety program. Annual report 2016
Implementing NAT& Universal Parasitic Screening for Blood Donors o The screening of blood in our country is not strictly regulated. o Safety of blood products: A great challenge o Limited resources & a high prevalence of HBV & HCV. o WHO recommends: syphilis and malaria in the basic blood screening criteria o NAT is performed in 06 centers only Kazi BM. Standards and guidelines for blood transfusion services. Islamabad: Pakistan NIH, Federal Health Ministry, Go. P Pakistan; 1999.

Implementing NAT & Universal Parasitic Screening for Blood Donors Shifa International Hospital q 550 bedded q. Tertiary care Hospital q. Cardiac Center q. Oncology Center q. Dialysis Center q. Orthopaedic Center q. Kidney, Liver & Bone marrow transplant q Gynae & Obs. Clinic

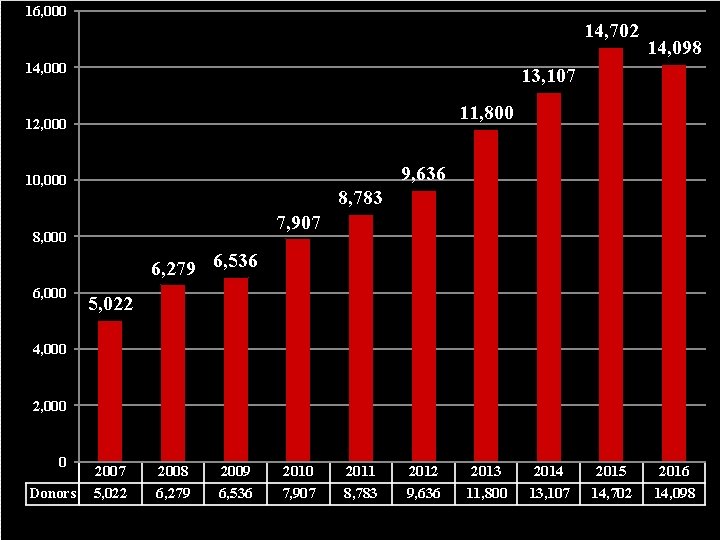
16, 000 14, 702 14, 000 14, 098 13, 107 11, 800 12, 000 9, 636 10, 000 8, 783 7, 907 8, 000 6, 279 6, 536 6, 000 5, 022 4, 000 2, 000 0 Donors 2007 5, 022 2008 6, 279 2009 6, 536 2010 7, 907 2011 8, 783 2012 9, 636 2013 11, 800 2014 13, 107 2015 14, 702 2016 14, 098
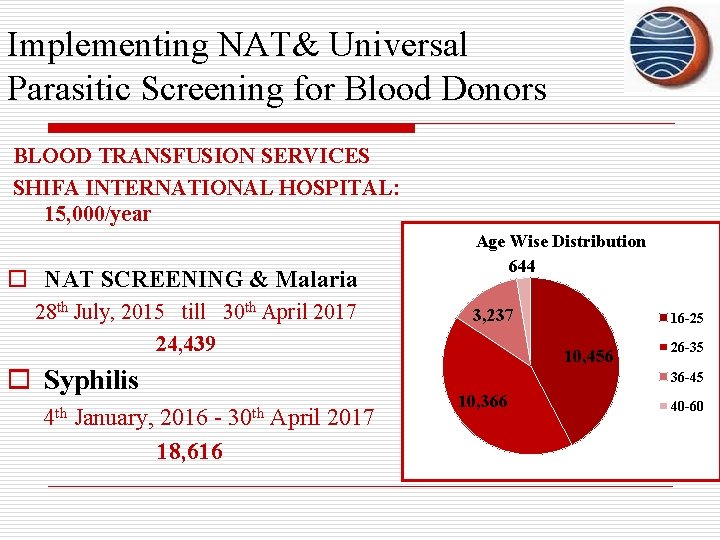
Implementing NAT& Universal Parasitic Screening for Blood Donors BLOOD TRANSFUSION SERVICES SHIFA INTERNATIONAL HOSPITAL: 15, 000/year o NAT SCREENING & Malaria 28 th July, 2015 till 30 th April 2017 24, 439 o Syphilis 4 th January, 2016 18, 616 Age Wise Distribution 644 3, 237 16 -25 10, 456 26 -35 36 -45 30 th April 2017 10, 366 40 -60

Implementing NAT& Universal Parasitic Screening for Blood Donors o Universal malaria Screening o BTS-SIH has started on ICT MP screening test on all blood donations 2015 o Initially started for liver transplant in 2012
Implementing NAT& Universal Parasitic Screening for Blood Donors Syphilis as TTI by WHO: 173 countries: policy of performing syphilis testing for all donations o 12 million new cases of syphilis each year, with >90% occurring in developing countries o In the past 30 years, through its association with an increased risk of HIV infection, syphilis has acquired a new potential for morbidity and mortality World Health Organization. Global prevalence and incidence of selected curable sexually transmitted infections: overview and estimates. 2001. [Accessed on 14/05/2014]. Available at: http: //www. who. int/hiv/pub/sti/en/who_hiv_aids_201405. pdf.
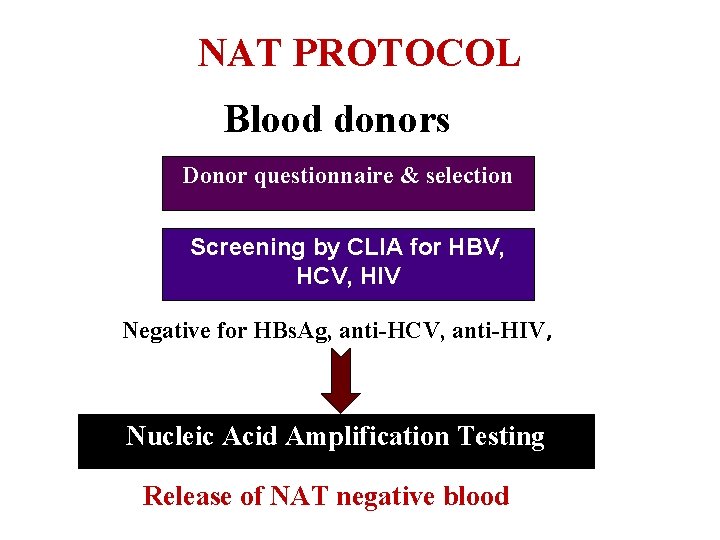
NAT PROTOCOL Blood donors DNA & RNA testing of HBV, HCV and HIV period infections Donor. Window questionnaire & selection Screening by CLIA for HBV, HCV, HIV Negative for HBs. Ag, anti-HCV, anti-HIV, Nucleic Acid Amplification Testing Release of NAT negative blood

Implementing NAT& Universal Parasitic Screening for Blood Donors Serological Screening o ROCHE cobas e 601 o Qualitative testing o CLIA based o Window period Nonspecific binding

Implementing NAT& Universal Parasitic Screening for Blood Donors o Initial sample is below the cutoff : Negative o If the sample exceeds that of the cutoff: Initially Reactive. o All IR are retested in duplicate o If one or both of the duplicate tests are also reactive: repeat reactive. . Positive. o A Positive Screening Test : DISCARD

Implementing NAT& Universal Parasitic Screening for Blood Donors Cobas Taq Screen MPX Test, v 2. 0 q Real-time, PCR technology q Four channel multi dye detection q Real time virus detection in blood plasma (mini pool of 6 samples) simultaneously detected and discriminated for HIV, HCV and HBV

Implementing NAT& Universal Parasitic Screening for Blood Donors MP-NAT : Cost-effective, comprehensive viral genotype Limitations: o The whole pooled donations is blocked until the NAT report is available. o Viral nucleic acid concentration gets diluted in the large pool of samples, the sensitivity of NAT might decrease o if a pool is tested reactive, the whole pool requires resolution to identify the single positive unit. an additional step.

Implementing NAT& Universal Parasitic Screening for Blood Donors: NAT SCREENING PROTOCOL In the case of reactivity by NAT, further NAT testing of an alternate sample source the plasma component bag or follow-up sampling of the donor is needed to confirm the test results.
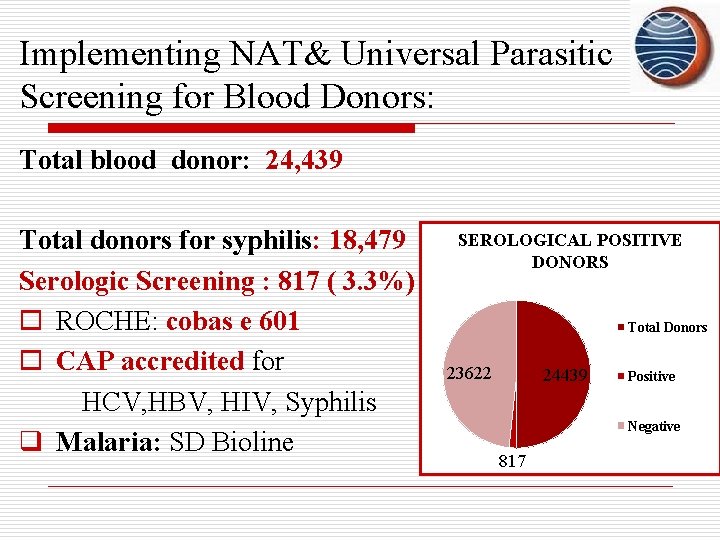
Implementing NAT& Universal Parasitic Screening for Blood Donors: Total blood donor: 24, 439 Total donors for syphilis: 18, 479 Serologic Screening : 817 ( 3. 3%) o ROCHE: cobas e 601 o CAP accredited for HCV, HBV, HIV, Syphilis q Malaria: SD Bioline SEROLOGICAL POSITIVE DONORS Total Donors 23622 24439 Positive Negative 817
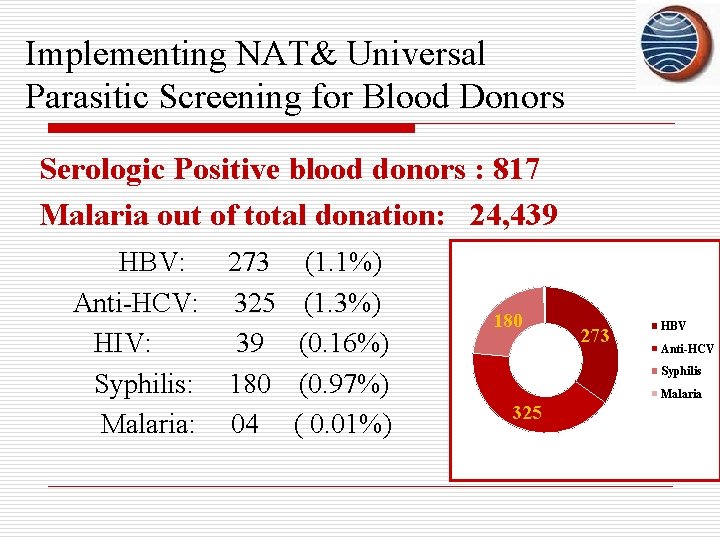
Implementing NAT& Universal Parasitic Screening for Blood Donors Serologic Positive blood donors : 817 Malaria out of total donation: 24, 439 HBV: Anti-HCV: HIV: Syphilis: Malaria: 273 (1. 1%) 325 (1. 3%) 39 (0. 16%) 180 (0. 97%) 04 ( 0. 01%) 180 273 HBV Anti-HCV Syphilis Malaria 325

Implementing NAT& Universal Parasitic Screening for Blood Donors Total NAT tested : 23, 622 o Minipool : 06 2 o HBV: o HCV: o HIV: 10 ( 0. 04%) 02 ( 0. 008%) NIL HBV HCV HIV 10
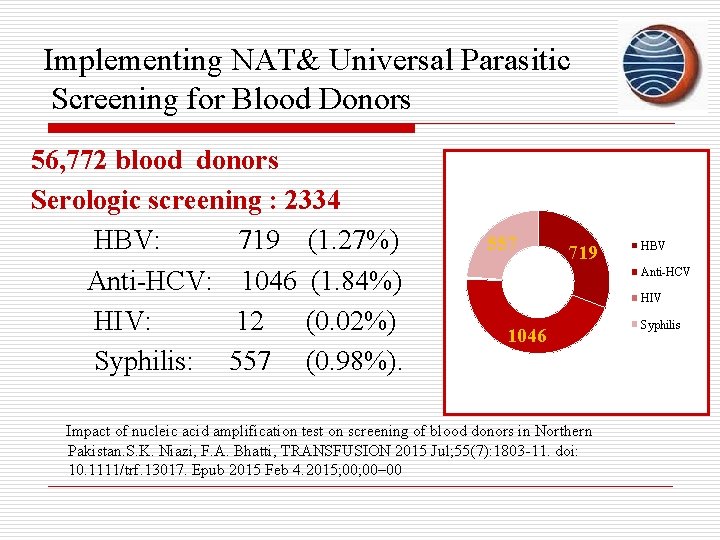
Implementing NAT& Universal Parasitic Screening for Blood Donors 56, 772 blood donors Serologic screening : 2334 HBV: 719 (1. 27%) Anti-HCV: 1046 (1. 84%) HIV: 12 (0. 02%) Syphilis: 557 (0. 98%). 557 719 HBV Anti-HCV HIV 1046 Impact of nucleic acid amplification test on screening of blood donors in Northern Pakistan. S. K. Niazi, F. A. Bhatti, TRANSFUSION 2015 Jul; 55(7): 1803 -11. doi: 10. 1111/trf. 13017. Epub 2015 Feb 4. 2015; 00– 00 Syphilis
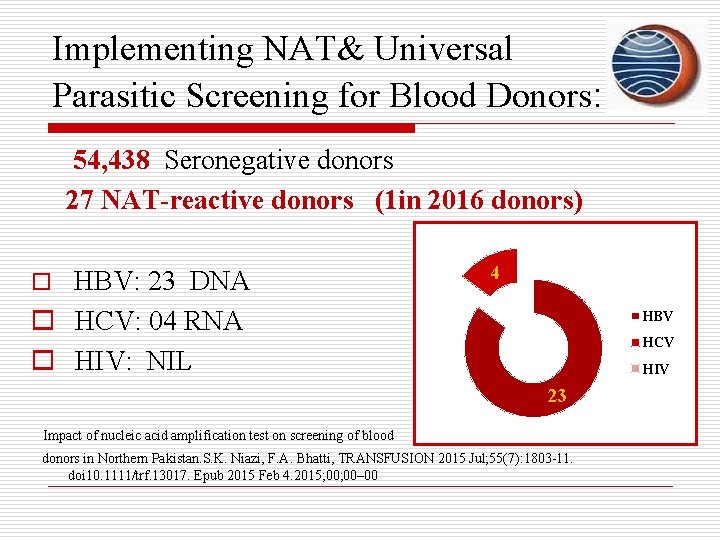
Implementing NAT& Universal Parasitic Screening for Blood Donors: 54, 438 Seronegative donors 27 NAT-reactive donors (1 in 2016 donors) o HBV: 23 DNA 4 o HCV: 04 RNA o HIV: NIL HBV HCV HIV 23 Impact of nucleic acid amplification test on screening of blood donors in Northern Pakistan. S. K. Niazi, F. A. Bhatti, TRANSFUSION 2015 Jul; 55(7): 1803 -11. doi 10. 1111/trf. 13017. Epub 2015 Feb 4. 2015; 00– 00
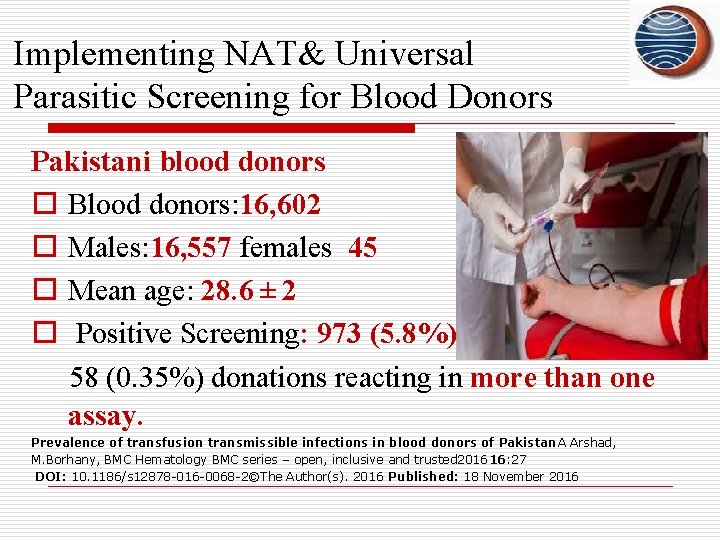
Implementing NAT& Universal Parasitic Screening for Blood Donors Pakistani blood donors o Blood donors: 16, 602 o Males: 16, 557 females 45 o Mean age: 28. 6 ± 2 o Positive Screening: 973 (5. 8%) 58 (0. 35%) donations reacting in more than one assay. Prevalence of transfusion transmissible infections in blood donors of Pakistan. A Arshad, M. Borhany, BMC Hematology BMC series – open, inclusive and trusted 201616: 27 DOI: 10. 1186/s 12878 -016 -0068 -2©The Author(s). 2016 Published: 18 November 2016
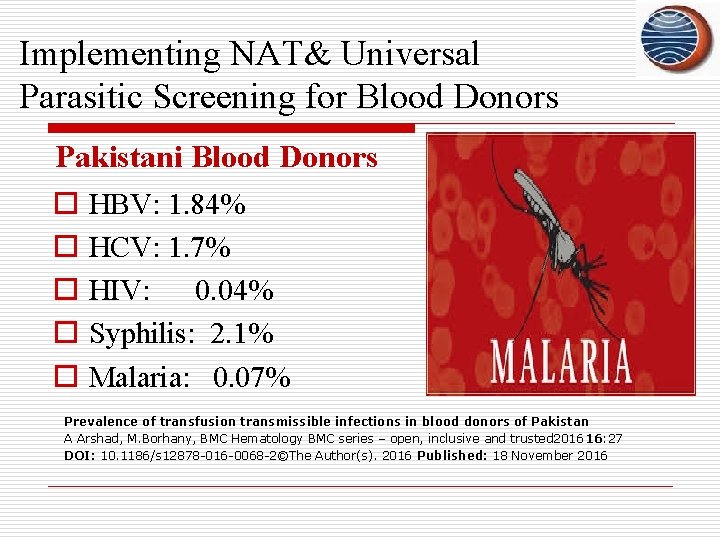
Implementing NAT& Universal Parasitic Screening for Blood Donors Pakistani Blood Donors o HBV: 1. 84% o HCV: 1. 7% o HIV: 0. 04% o Syphilis: 2. 1% o Malaria: 0. 07% Prevalence of transfusion transmissible infections in blood donors of Pakistan A Arshad, M. Borhany, BMC Hematology BMC series – open, inclusive and trusted 2016 16: 27 DOI: 10. 1186/s 12878 -016 -0068 -2©The Author(s). 2016 Published: 18 November 2016
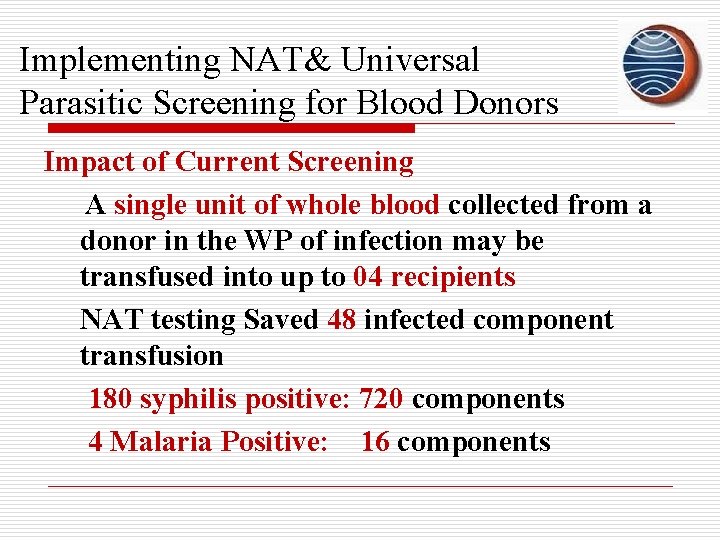
Implementing NAT& Universal Parasitic Screening for Blood Donors Impact of Current Screening A single unit of whole blood collected from a donor in the WP of infection may be transfused into up to 04 recipients NAT testing Saved 48 infected component transfusion 180 syphilis positive: 720 components 4 Malaria Positive: 16 components

Implementing NAT& Universal Parasitic Screening for Blood Donors Future Tasks: Ø Ø Ø JCIA Inspection CAP accreditation ISBT 128 X-ray Irradiator Transfusion Nurses Shift: Replacement to voluntary donation

Implementing NAT& Universal Parasitic Screening for Blood Donors ISBT 128: Gobal standard for o Identification o labeling o Information transfer of medical products of human origin (blood, cells, tissues, milk, and organ products) across international borders and disparate health care systems

THANK YOU
- Slides: 39