Implementing a LaboratoryBased Rapid HIV Testing Algorithm using

Implementing a Laboratory-Based Rapid HIV Testing Algorithm using Two Different Test Kits in a Hospital Emergency Department Jason S. Haukoos 1, MD, MSc, Emily Hopkins 1, MSPH, Brian Boyett 2, MS, Kevin P. Delaney 2, MPH 1 Denver Health Medical Center, Denver, Colorado 2 Centers for Disease Control and Prevention, Atlanta, Georgia 2007 HIV Diagnostics Conference December 6, 2007
Background • The CDC currently recommends performing nontargeted, opt-out rapid HIV screening in healthcare settings with prevalences ≥ 0. 1%, including emergency departments (EDs) • With over 110 million ED visits in the United States each year, this may result in large numbers of patients being tested and a relatively large number of patients who test positive

Background • The specificities of current FDA-approved rapid tests range from 98. 6%– 100% • Such high levels of screening may result in a relatively large number of false-positive results
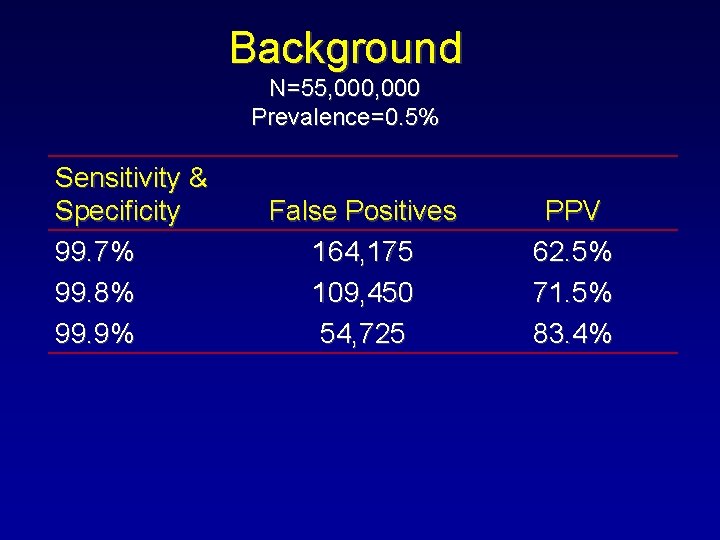
Background N=55, 000 Prevalence=0. 5% Sensitivity & Specificity 99. 7% 99. 8% 99. 9% False Positives 164, 175 109, 450 54, 725 PPV 62. 5% 71. 5% 83. 4%

Background • Currently, the CDC recommends that all preliminary positive rapid tests be confirmed with a Western blot • In order to more quickly distinguish persons likely to have false-positive test results, multiple rapid testing algorithms have been proposed

Objective • To evaluate a laboratory-based, multiple rapid HIV testing algorithm as part of a controlled clinical trial to evaluate the effectiveness and efficiency of performing non-targeted, opt-out rapid HIV screening in an urban ED

Methods • Non-targeted, opt-out rapid HIV testing was implemented in the ED at Denver Health Medical Center in Denver, Colorado – Urban, safety-net hospital and level 1 trauma center serving the City and County of Denver, Colorado – Approximately 55, 000 adults seek care annually – Estimated HIV seroprevalence: 0. 7%
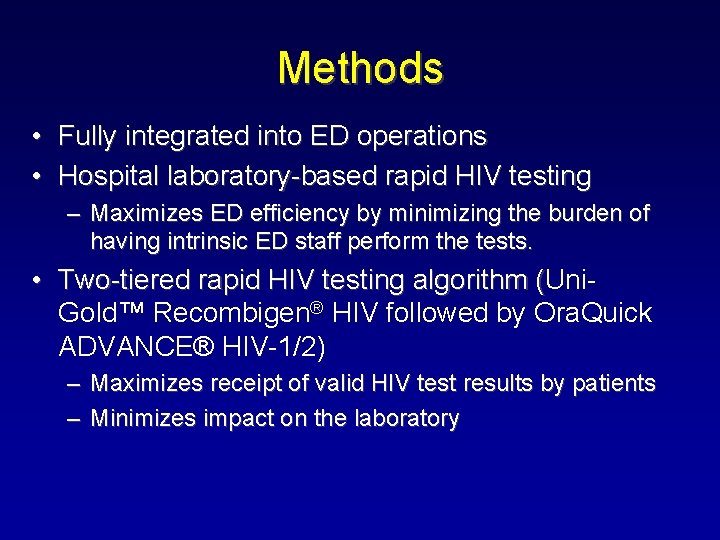
Methods • Fully integrated into ED operations • Hospital laboratory-based rapid HIV testing – Maximizes ED efficiency by minimizing the burden of having intrinsic ED staff perform the tests. • Two-tiered rapid HIV testing algorithm (Uni( Gold™ Recombigen® HIV followed by Ora. Quick ADVANCE® HIV-1/2) – Maximizes receipt of valid HIV test results by patients – Minimizes impact on the laboratory

Methods • All test results (i. e. , negative, concordant positive, or discordant positive) reported by the hospital laboratory within approximately one hour using the standard electronic laboratory reporting system • All patients who tested positive (concordant or discordant) for HIV infection provided posttest counseling and linked to care for confirmatory testing – Concordant positives: Western blot performed at state laboratory – Discordant positives: Multispot® HIV-1/HIV-2 and Western blot performed at state laboratory
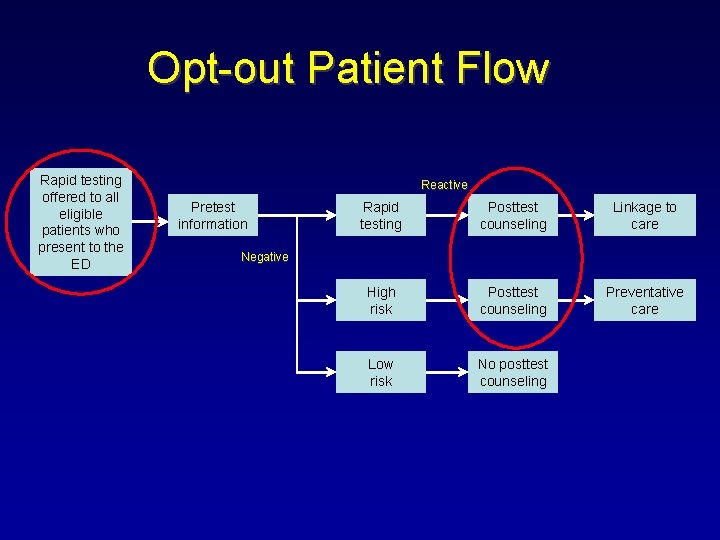
Opt-out Patient Flow Rapid testing offered to all eligible patients who present to the ED Reactive Pretest information Rapid testing Posttest counseling Linkage to care High risk Posttest counseling Preventative care Low risk No posttest counseling Negative
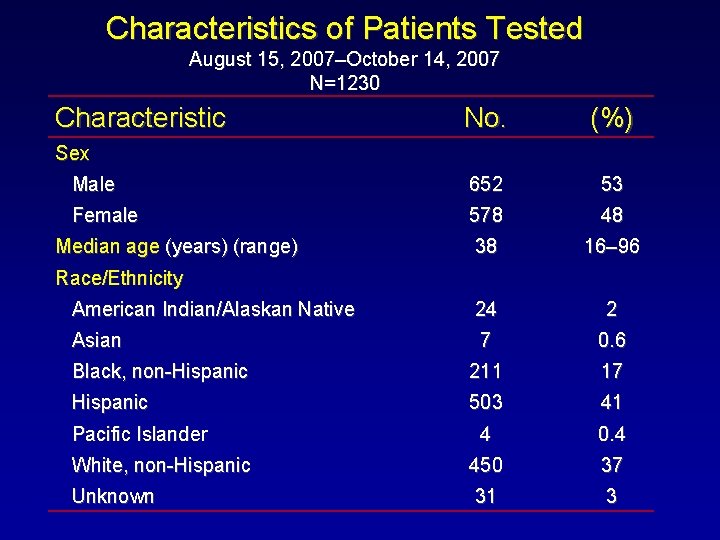
Characteristics of Patients Tested August 15, 2007–October 14, 2007 N=1230 Characteristic No. (%) Male 652 53 Female 578 48 38 16– 96 American Indian/Alaskan Native 24 2 Asian 7 0. 6 Black, non-Hispanic 211 17 Hispanic 503 41 4 0. 4 White, non-Hispanic 450 37 Unknown 31 3 Sex Median age (years) (range) Race/Ethnicity Pacific Islander
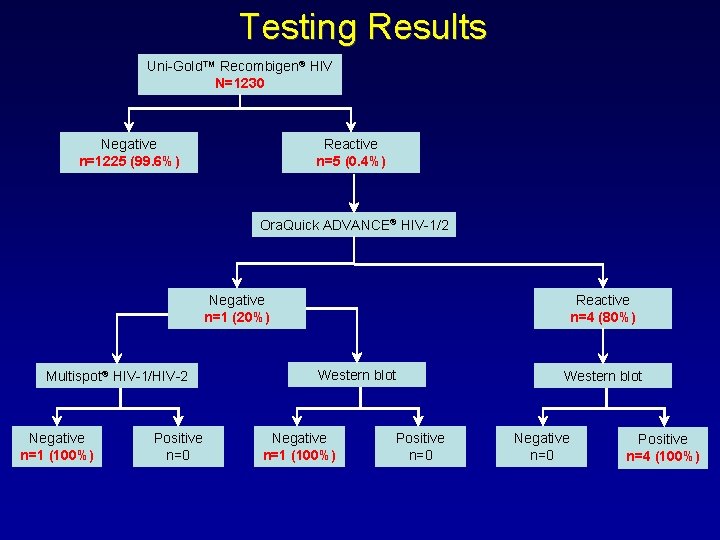
Testing Results Uni-Gold™ Recombigen® HIV N=1230 Negative n=1225 (99. 6%) Reactive n=5 (0. 4%) Ora. Quick ADVANCE® HIV-1/2 Negative n=1 (20%) Multispot® HIV-1/HIV-2 Negative n=1 (100%) Positive n=0 Reactive n=4 (80%) Western blot Negative n=1 (100%) Positive n=0 Western blot Negative n=0 Positive n=4 (100%)

Conclusions • Partnering with our hospital laboratory allowed us to implement a two-tiered rapid HIV testing algorithm in our ED • This enabled us to provide more information to both the hospital staff and the patients who had positive preliminary test results • Although diagnostic experience with a larger number of patients will be required to make definitive recommendations, a two-tiered rapid HIV testing algorithm holds promise for rapid HIV testing in the ED setting

Funding Agency Division of HIV/AIDS Prevention National Center for HIV, STD, and TB Prevention Centers for Disease Control and Prevention Atlanta, Georgia U 18 PS 000314 September 1, 2006 – August 31, 2008 Contact Information Jason Haukoos, MD, MSc jason. haukoos@dhha. org Emily Hopkins, MSPH emily. hopkins@dhha. org

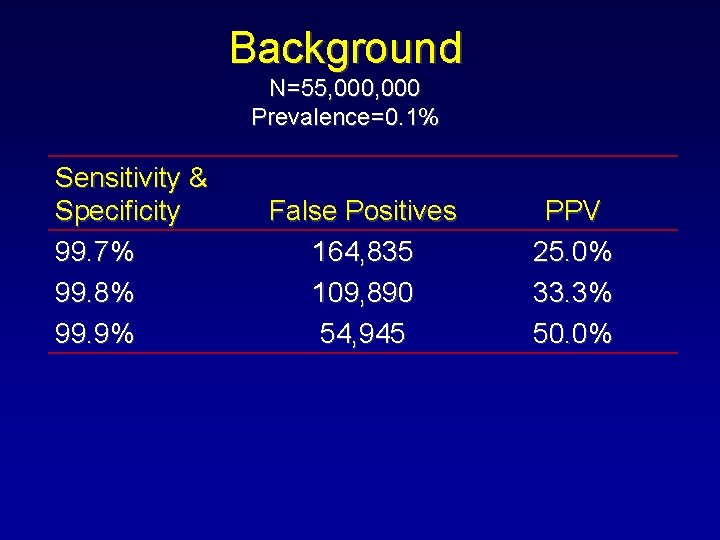
Background N=55, 000 Prevalence=0. 1% Sensitivity & Specificity 99. 7% 99. 8% 99. 9% False Positives 164, 835 109, 890 54, 945 PPV 25. 0% 33. 3% 50. 0%
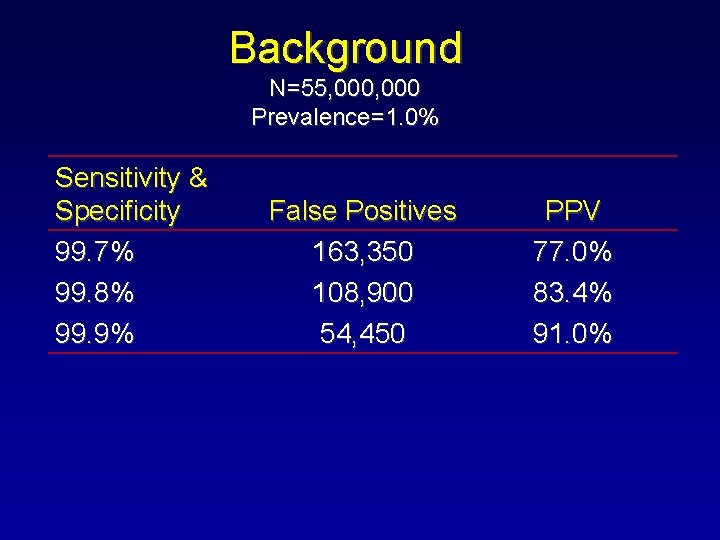
Background N=55, 000 Prevalence=1. 0% Sensitivity & Specificity 99. 7% 99. 8% 99. 9% False Positives 163, 350 108, 900 54, 450 PPV 77. 0% 83. 4% 91. 0%
- Slides: 17