IMPLEMENTATION OF MEDICAL DECISION MAKING SYSTEM BY CLASSIFICATION
IMPLEMENTATION OF MEDICAL DECISION MAKING SYSTEM BY CLASSIFICATION OF ULTRASOUND COMMON CAROTID ARTERY IMAGES USING INTIMA MEDIA THICKNESS MEASUREMENT Dr. N. Santhiyakumari Prof & Head/ECE Knowledge Institute of technology, Salem

2 MOTIVATION A recent report of world health organization (WHO) has predicted that cardiovascular disease will become the number one cause of mortality globally by the year 2015 and will account for an estimated 20 million people death annually.

3 PROBLEM IDENTIFICATION Cardiovascular disease (CVD) and cerebrovascular disease affects millions of people in the world. It has been reported that CVD remains the leading cause of death in India in the recent past.

4 TECHNOLOGIES X-rays Magnetic Resonance Imaging (MRI) Magnetic Resonance Angiography (MRA) Computed Tomography (CT) Positron Emission Tomography (PET) Single Photon Emission Tomography (SPECT) Ultrasonography (US)

5 REASON Even though there has been some improvement in cardiovascular disease assessment and intervention in men, there has been little progress regarding the detection and prevention of CVD and cerebrovascular disease. If a system could be automated to track the thickening of the vessel wall to predict the pathology, it would be a milestone in the efforts taken to prevent CVD and cerebrovascular disease.
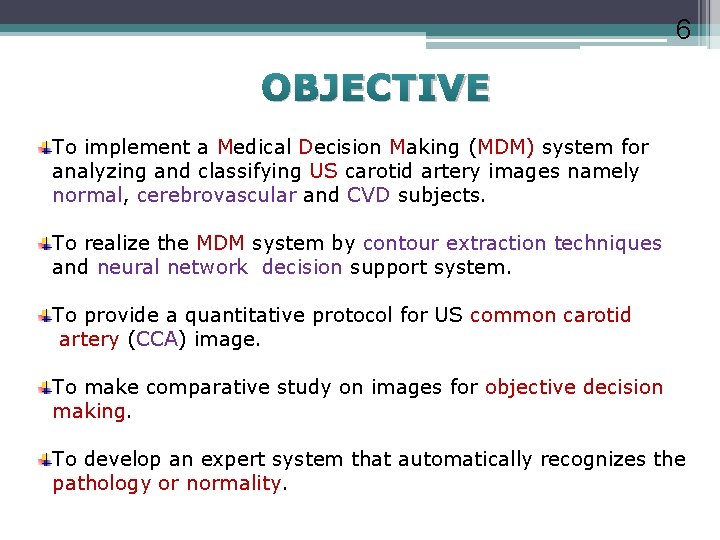
6 OBJECTIVE To implement a Medical Decision Making (MDM) system for analyzing and classifying US carotid artery images namely normal, cerebrovascular and CVD subjects. To realize the MDM system by contour extraction techniques and neural network decision support system. To provide a quantitative protocol for US common carotid artery (CCA) image. To make comparative study on images for objective decision making. To develop an expert system that automatically recognizes the pathology or normality.

7 PROPOSED METHOD Ultrasound Images Of The Blood Vessel are used. Images Are Taken In B-mode. One Cardiac Cycle is enough. (1 Systole & Diastole). Patients With Hypertension & Diabetes are expected to have greater Thickness. Why carotid artery? Recent studies shows that problems in carotid artery are reflected in other arteries.
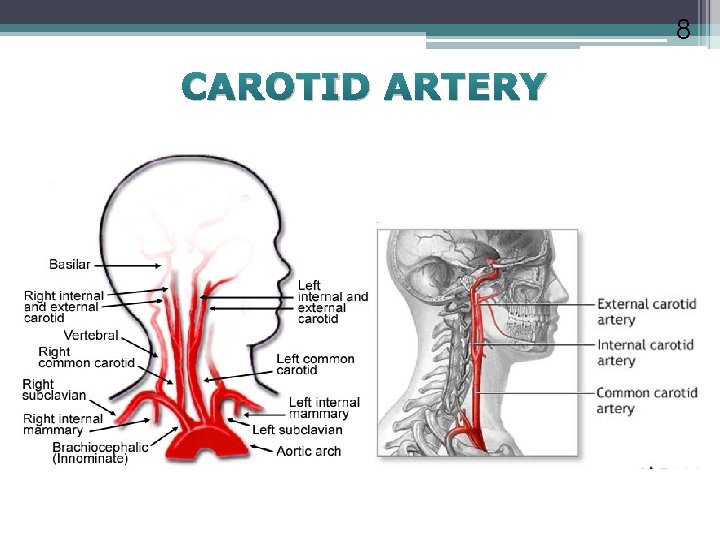
8 CAROTID ARTERY
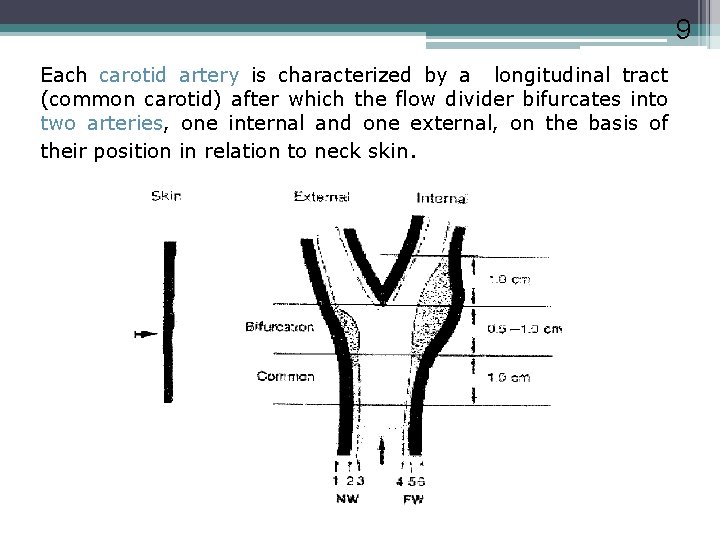
9 Each carotid artery is characterized by a longitudinal tract (common carotid) after which the flow divider bifurcates into two arteries, one internal and one external, on the basis of their position in relation to neck skin.
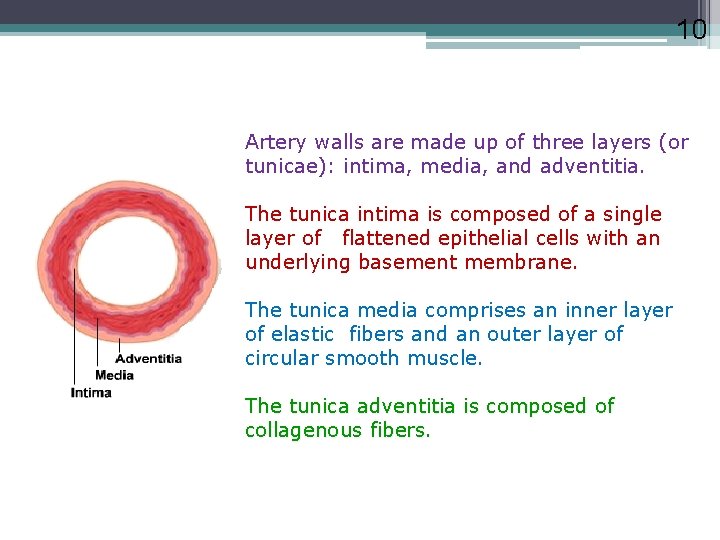
10 Artery walls are made up of three layers (or tunicae): intima, media, and adventitia. The tunica intima is composed of a single layer of flattened epithelial cells with an underlying basement membrane. The tunica media comprises an inner layer of elastic fibers and an outer layer of circular smooth muscle. The tunica adventitia is composed of collagenous fibers.
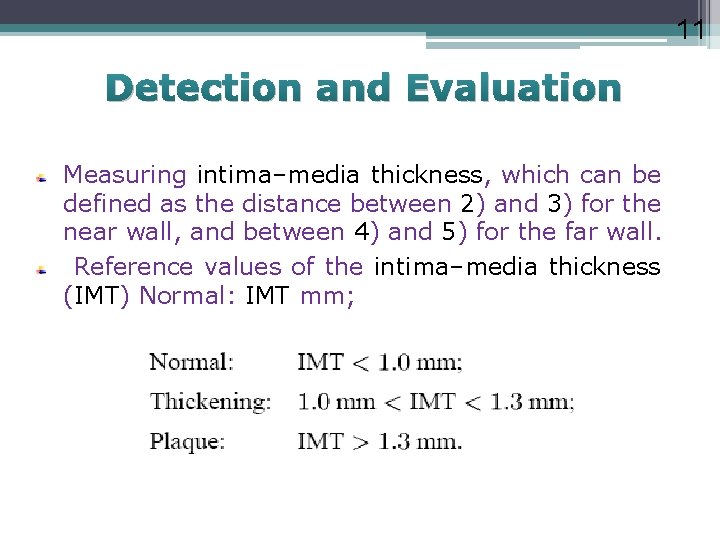
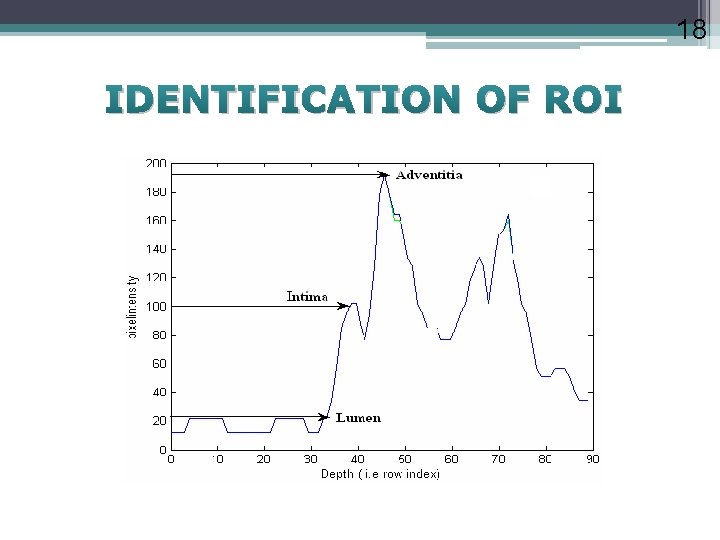
11 Detection and Evaluation Measuring intima–media thickness, which can be defined as the distance between 2) and 3) for the near wall, and between 4) and 5) for the far wall. Reference values of the intima–media thickness (IMT) Normal: IMT mm;

12 THICKENING Genetics and environmental factors combine over time to cause inflammation of the inner layers of the artery and the formation of plaque on the inner lineing of the arterial wall. This thickening can be measured using ultrasound and sophisticated edge-detection software to quantify the amount of disease present. Measurement of the thickness of the intima and media layers of the common carotid artery is predictive of future events (i. e. stroke, myocardial infarction and heart attack)
13 INTIMA-MEDIA TEST It is a painless non-invasive test (using digital ultrasound technology and digital edge detection sophisticated software). The Carotid Intima-Media Thickness (CIMT) scan is brief (approximately 10 minutes), does not require the patient to disrobe, is noninvasive (no needles), and does not expose the patient to radiation. It is relatively inexpensive and provides valuable information about an individual's risk of experiencing a heart attack, stroke, or MI.
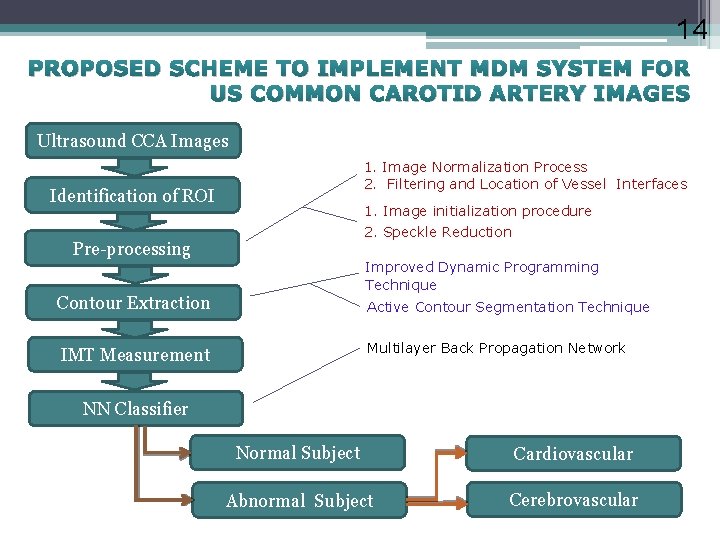
14 PROPOSED SCHEME TO IMPLEMENT MDM SYSTEM FOR US COMMON CAROTID ARTERY IMAGES Ultrasound CCA Images 1. Image Normalization Process 2. Filtering and Location of Vessel Interfaces Identification of ROI 1. Image initialization procedure 2. Speckle Reduction Pre-processing Improved Dynamic Programming Technique Contour Extraction Active Contour Segmentation Technique Multilayer Back Propagation Network IMT Measurement NN Classifier Normal Subject Cardiovascular Abnormal Subject Cerebrovascular
15 IMAGE ACQUISTION Ultrasound Imaging system Ø Aloka Prosound Alpha -10 (SSD α -10, Model No-M 00720 Japan) Multifrequency probe(5 -10)MHz and Frequency fixed at 7. 5 MHz Ø Philips HD 11 XE US machine (Model No – HDI 5000 sonoct) Multifrequency probe(7 -15)MHz and Frequency fixed at 12 MHz Common Carotid Artery’s longitudinal image as well as transversal image in B-mode. Recording is done 2 cm before the bifurcation of the Common Carotid Artery to maintain uniformity in measurement. Blood Pressure is checked and recorded. ECG probes are placed in radial artery and posterior tibial artery.
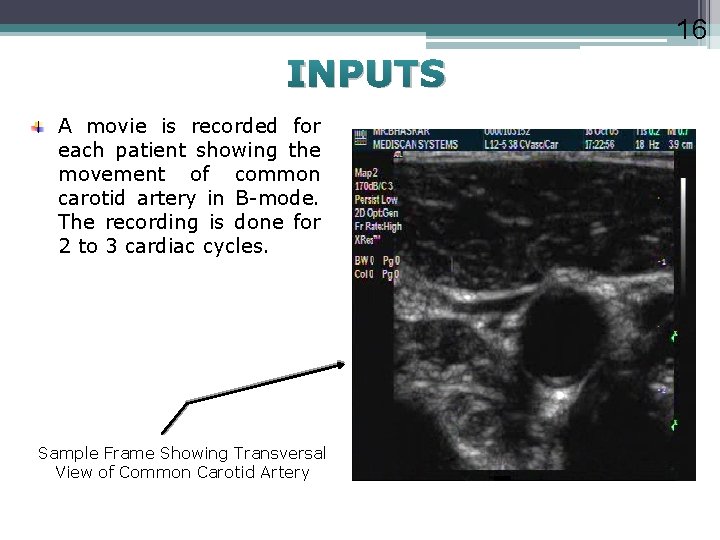
16 INPUTS A movie is recorded for each patient showing the movement of common carotid artery in B-mode. The recording is done for 2 to 3 cardiac cycles. Sample Frame Showing Transversal View of Common Carotid Artery
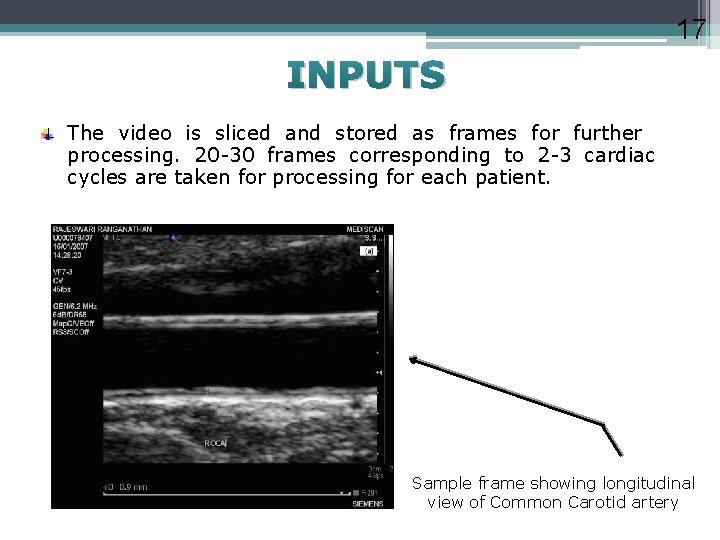
17 INPUTS The video is sliced and stored as frames for further processing. 20 -30 frames corresponding to 2 -3 cardiac cycles are taken for processing for each patient. Sample frame showing longitudinal view of Common Carotid artery
18 IDENTIFICATION OF ROI
19 PREPROCESSING OF IMAGES v. Image Normalization Process v. Filtering and Location of Vessel Interfaces üImage initialization procedure üSpeckle Reduction
20 Image Normalization Process Histogram contrast information and overall intensity distribution. Intensity normalization average intensity histogram Filtering Smoothing filter 2 D median filter (3× 3 square filter) Removes speckles and preserves edges Location of Vessel Interfaces Horizontal starting position Identified ROI Vertical starting position 5× 5 plus sign shaped median filter
21 Image initialization procedure selecting upper left vertex P 1(x 1, y 1) lower right vertex P 2(x 2, y 2) Speckle Reduction Model the noise Gaussian noise with zero mean Speckle reducing anisotropic diffusion method (SRAD) Enhances the edges by inhibiting diffusion across edges and allowing diffusion on either side of the edge. SRADPDE Reducing speckle and provides superior performance over conventional anisotropic diffusion method in terms of smoothing uniform regions and preserving edges and features.

22 Contour Extraction Techniques Improved Dynamic Programming Technique Active Contour Segmentation Technique
23 Improved Dynamic Programming Technique Determination of the Cost function for each point in the polylines of identified region. Estimation of the boundaries by optimizing the cost function. Measurement of the distance between the intimal and medial layers of the far wall of identified region of the image. Evaluation the IMT of various subjects.
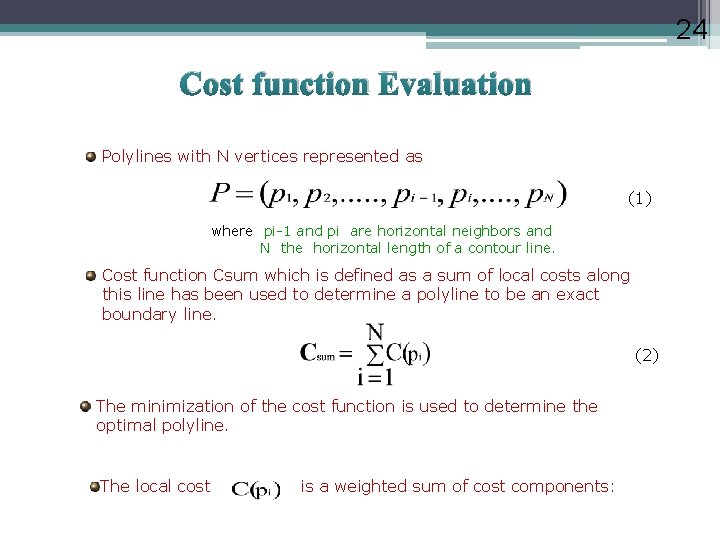
24 Cost function Evaluation Polylines with N vertices represented as (1) where pi-1 and pi are horizontal neighbors and N the horizontal length of a contour line. Cost function Csum which is defined as a sum of local costs along this line has been used to determine a polyline to be an exact boundary line. (2) The minimization of the cost function is used to determine the optimal polyline. The local cost is a weighted sum of cost components:

25 (3) Where , , are weighting factors is the value of polylines discontinuity (measure of smoothness), is the average brightness below the tested pixel and is the value of the intensity gradient (the rate of change of the intensity). The values for the weight factors have been utilized as W 1+W 2+W 3=1 Two of the weight vector values are used in steps of 0. 09, such that W 1+W 2<1 The weights W 1=0. 5 and W 2=0. 2 has been used in this technique
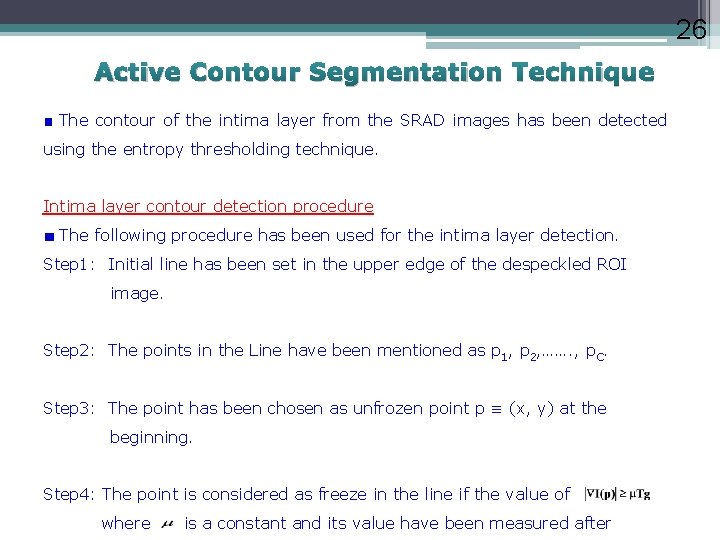
26 Active Contour Segmentation Technique The contour of the intima layer from the SRAD images has been detected using the entropy thresholding technique. Intima layer contour detection procedure The following procedure has been used for the intima layer detection. Step 1: Initial line has been set in the upper edge of the despeckled ROI image. Step 2: The points in the Line have been mentioned as p 1, p 2, ……. , p. C. Step 3: The point has been chosen as unfrozen point p ≡ (x, y) at the beginning. Step 4: The point is considered as freeze in the line if the value of where is a constant and its value have been measured after

27 several iterations as 0. 73 and Tg is a threshold of the image. Its value has been calculated from the histogram gradient of ROI through the entropy threshold method. Step 5: The point p has been fixed at the position if one of the neighbors of p is frozen with higher gradient between (x, y-1), (x, y) and (x, y-1), this step has been followed by step 7. Step 6: The point has been moved to (x, y+1). Step 7: If all the points are fixed, then the steps have been terminated. Step 8: If all the points are not fixed, then the step 3 has been followed.

28 Medial layer Contour detection procedure The following steps have been used to calculate the chain of points in a line. Step 1: The point L 0 has been set as L 1 = P 1(x 1, y 1). Step 2: For each and every point (i. e. , L 2, …. , Lc) in a line the location has been searched as Li = Li-1(xi-1+1, yi-1+j ) with j = -1, 0, 1 where the function has higher value and Li(xi, yi) satisfies the condition as Li(xi, yi-S) = pi (xi, yi) where S distance.

29 The effect of the intima layer is null in the energy minimization process since the value for a distance is a constant and this value has been considered as the intima thickness The initial contour defined by the above process avoids the multiple contour and it guarantees a fast convergence in the minimization process. In this way, the energy minimization process has not been affected by the intimal edge attraction.
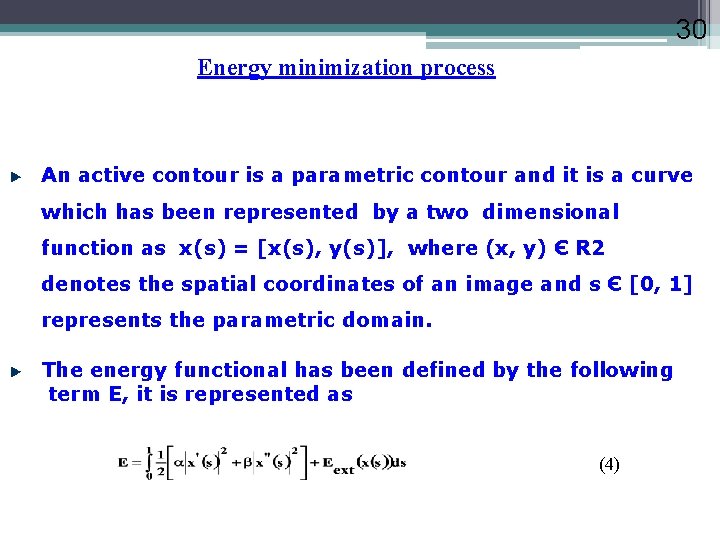
30 Energy minimization process An active contour is a parametric contour and it is a curve which has been represented by a two dimensional function as x(s) = [x(s), y(s)], where (x, y) Є R 2 denotes the spatial coordinates of an image and s Є [0, 1] represents the parametric domain. The energy functional has been defined by the following term E, it is represented as (4)
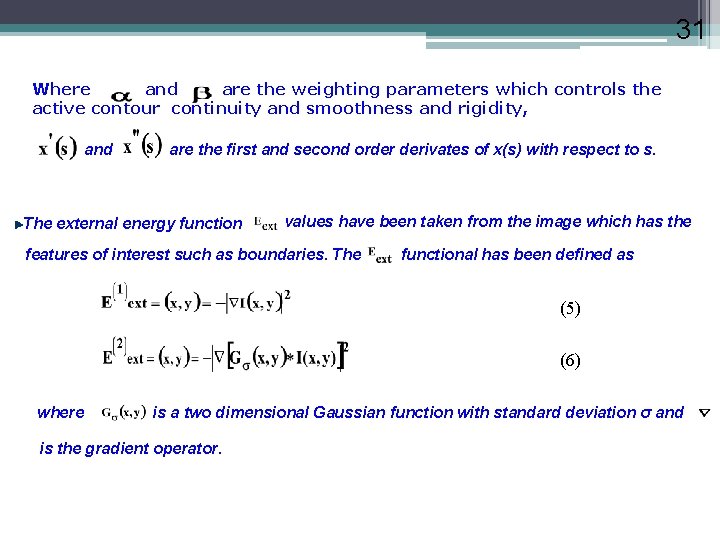
31 Where and are the weighting parameters which controls the active contour continuity and smoothness and rigidity, and are the first and second order derivates of x(s) with respect to s. The external energy function values have been taken from the image which has the features of interest such as boundaries. The functional has been defined as (5) (6) where is a two dimensional Gaussian function with standard deviation σ and is the gradient operator.

32 Large value of σ has been taken since this large value makes the boundary to be blurry one. The capture range of the active contour has been increased by energy minimization process which satisfies the following Euler equation. It is denoted as (7) This Euler expression can be represented as a force balance equation (8) Where = and = The values for the term α = 0. 55 and β= 0. 43 have been used in this work.
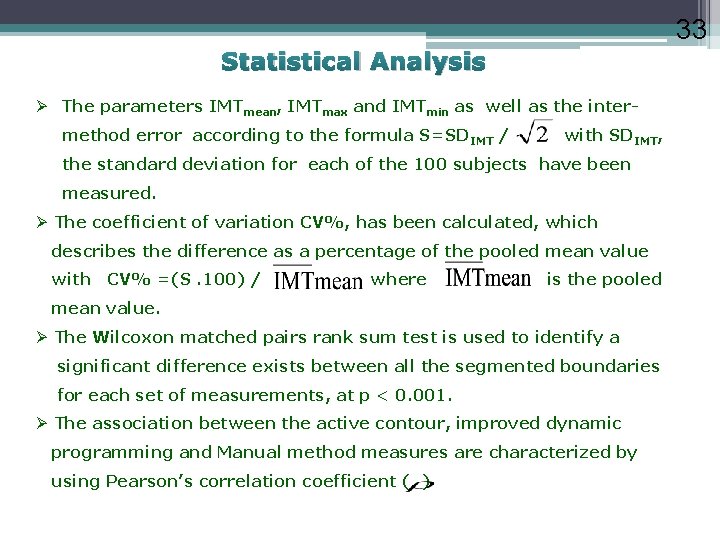
33 Statistical Analysis Ø The parameters IMTmean, IMTmax and IMTmin as well as the intermethod error according to the formula S=SDIMT / with SDIMT, the standard deviation for each of the 100 subjects have been measured. Ø The coefficient of variation CV%, has been calculated, which describes the difference as a percentage of the pooled mean value with CV% =(S. 100) / where is the pooled mean value. Ø The Wilcoxon matched pairs rank sum test is used to identify a significant difference exists between all the segmented boundaries for each set of measurements, at p < 0. 001. Ø The association between the active contour, improved dynamic programming and Manual method measures are characterized by using Pearson’s correlation coefficient ( )
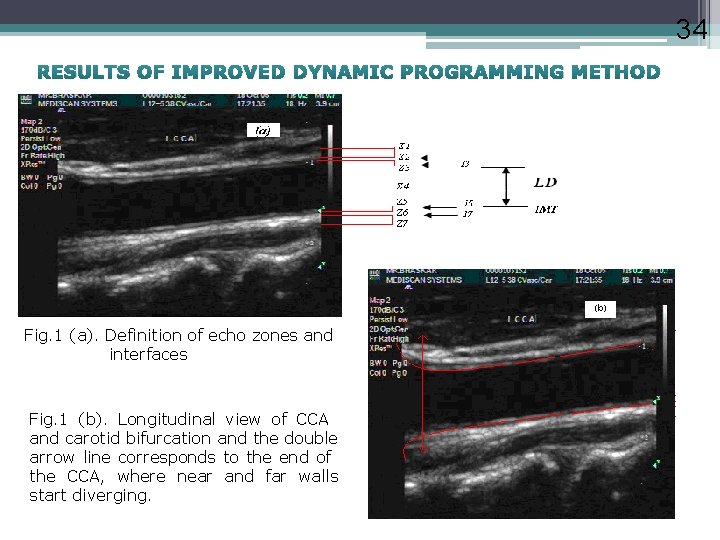
34 RESULTS OF IMPROVED DYNAMIC PROGRAMMING METHOD Fig. 1 (a). Definition of echo zones and interfaces Fig. 1 (b). Longitudinal view of CCA and carotid bifurcation and the double arrow line corresponds to the end of the CCA, where near and far walls start diverging.
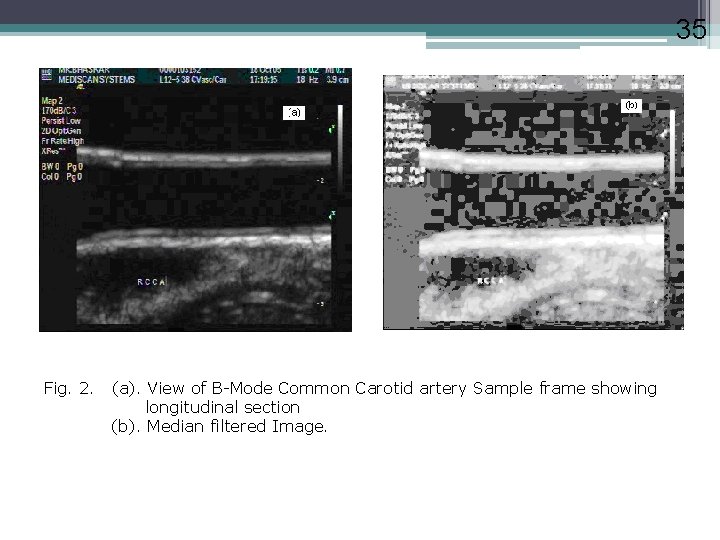
35 Fig. 2. (a). View of B-Mode Common Carotid artery Sample frame showing longitudinal section (b). Median filtered Image.
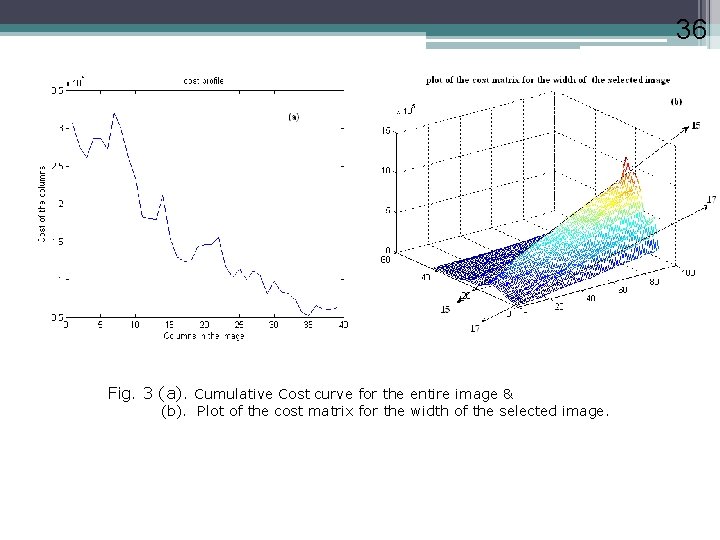
36 Fig. 3 (a). Cumulative Cost curve for the entire image & (b). Plot of the cost matrix for the width of the selected image. 36
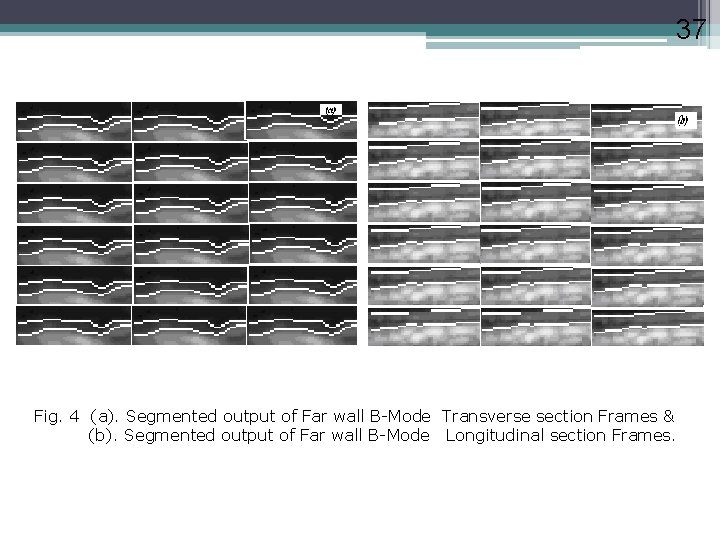
37 Fig. 4 (a). Segmented output of Far wall B-Mode Transverse section Frames & (b). Segmented output of Far wall B-Mode Longitudinal section Frames.
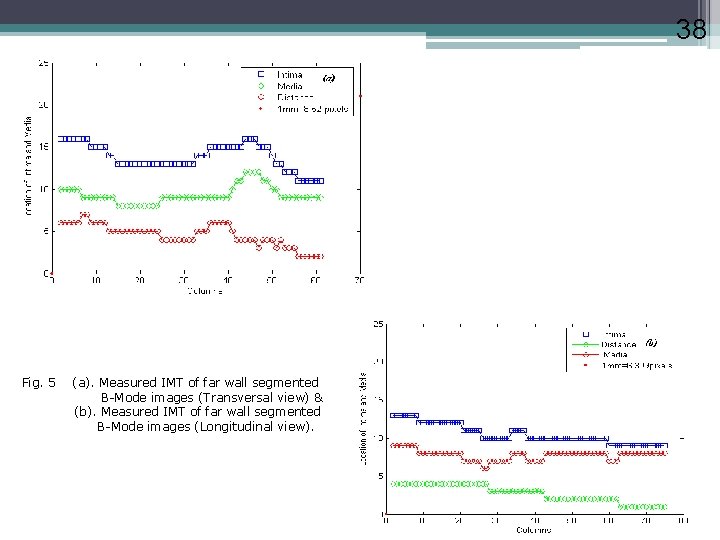
38 Fig. 5 (a). Measured IMT of far wall segmented B-Mode images (Transversal view) & (b). Measured IMT of far wall segmented B-Mode images (Longitudinal view).
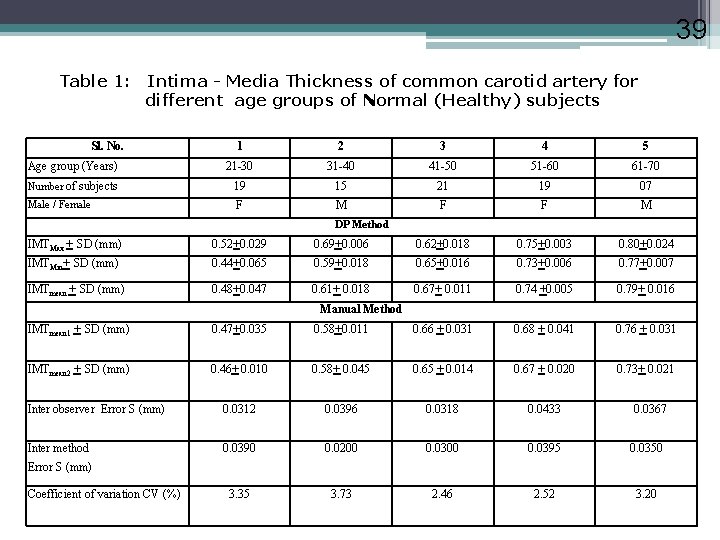
39 Table 1: Intima - Media Thickness of common carotid artery for different age groups of Normal (Healthy) subjects Sl. No. 1 2 3 4 5 Age group (Years) 21 -30 31 -40 41 -50 51 -60 61 -70 Number of subjects 19 15 21 19 07 Male / Female F M F F M DP Method IMTMax + SD (mm) 0. 52+0. 029 0. 69+0. 006 0. 62+0. 018 0. 75+0. 003 0. 80+0. 024 IMTMin+ SD (mm) 0. 44+0. 065 0. 59+0. 018 0. 65+0. 016 0. 73+0. 006 0. 77+0. 007 IMTmean + SD (mm) 0. 48+0. 047 0. 61+ 0. 018 0. 67+ 0. 011 0. 74 +0. 005 0. 79+ 0. 016 Manual Method IMTmean 1 + SD (mm) 0. 47+0. 035 0. 58+0. 011 0. 66 + 0. 031 0. 68 + 0. 041 0. 76 + 0. 031 IMTmean 2 + SD (mm) 0. 46+ 0. 010 0. 58+ 0. 045 0. 65 + 0. 014 0. 67 + 0. 020 0. 73+ 0. 021 Inter observer Error S (mm) 0. 0312 0. 0396 0. 0318 0. 0433 Inter method 0. 0390 0. 0200 0. 0395 0. 0350 3. 35 3. 73 2. 46 2. 52 3. 20 0. 0367 Error S (mm) Coefficient of variation CV (%)
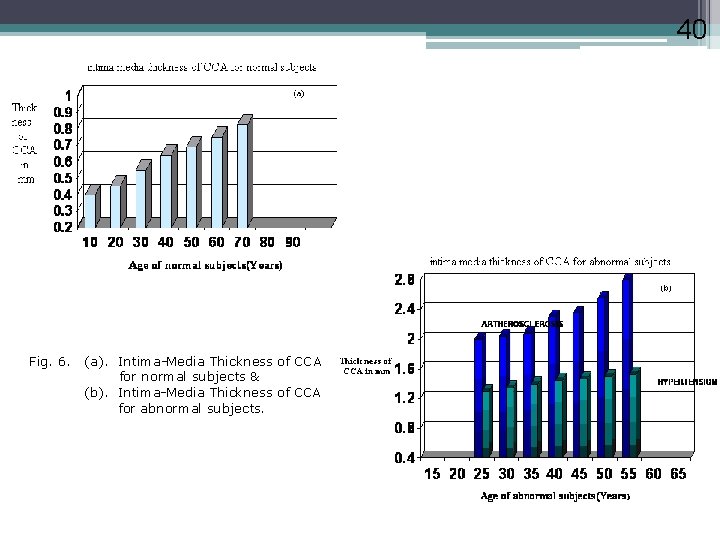
40 Fig. 6. (a). Intima-Media Thickness of CCA for normal subjects & (b). Intima-Media Thickness of CCA for abnormal subjects.
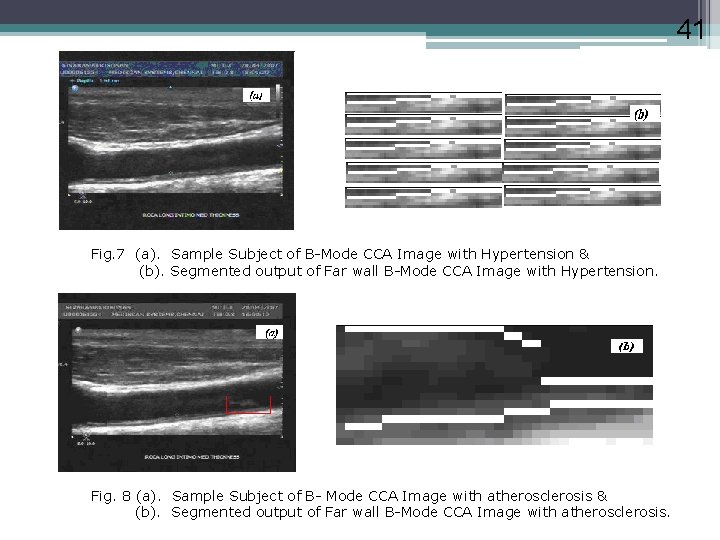
41 Fig. 7 (a). Sample Subject of B-Mode CCA Image with Hypertension & (b). Segmented output of Far wall B-Mode CCA Image with Hypertension. Fig. 8 (a). Sample Subject of B- Mode CCA Image with atherosclerosis & (b). Segmented output of Far wall B-Mode CCA Image with atherosclerosis.
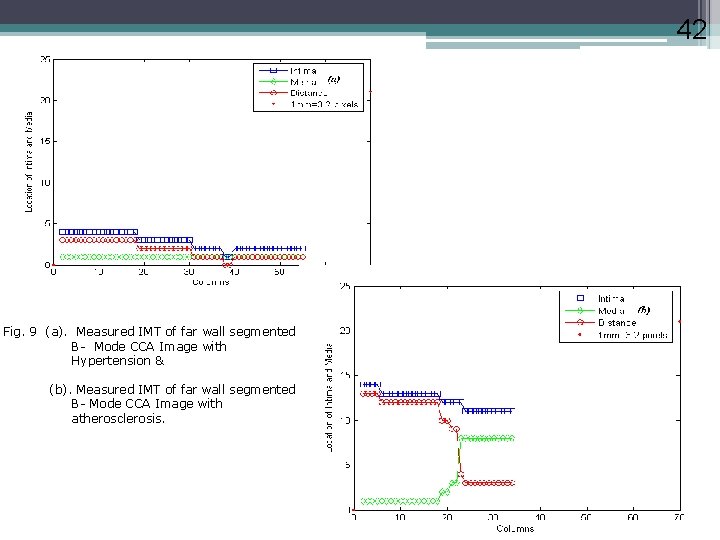
42 Fig. 9 (a). Measured IMT of far wall segmented B- Mode CCA Image with Hypertension & (b). Measured IMT of far wall segmented B- Mode CCA Image with atherosclerosis.
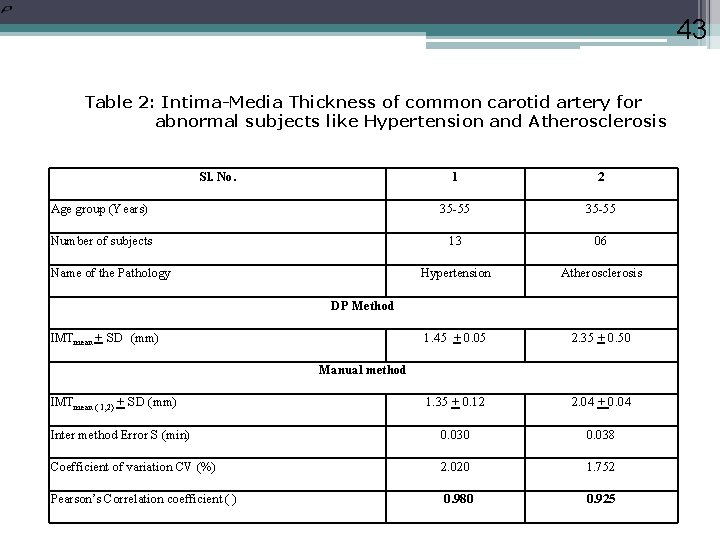
43 Table 2: Intima-Media Thickness of common carotid artery for abnormal subjects like Hypertension and Atherosclerosis Sl. No. 1 2 Age group (Years) 35 -55 Number of subjects 13 06 Hypertension Atherosclerosis 1. 45 + 0. 05 2. 35 + 0. 50 1. 35 + 0. 12 2. 04 + 0. 04 Inter method Error S (min) 0. 030 0. 038 Coefficient of variation CV (%) 2. 020 1. 752 Pearson’s Correlation coefficient ( ) 0. 980 0. 925 Name of the Pathology DP Method IMTmean + SD (mm) Manual method IMTmean ( 1, 2) + SD (mm)
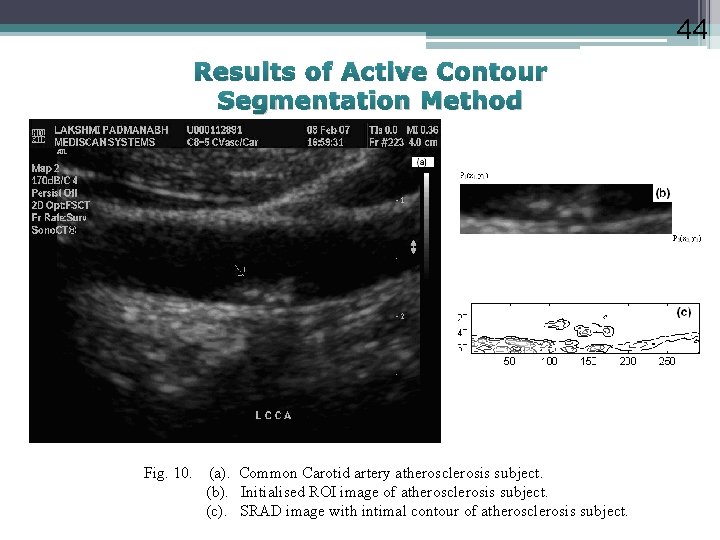
44 Results of Active Contour Segmentation Method Fig. 10. (a). Common Carotid artery atherosclerosis subject. (b). Initialised ROI image of atherosclerosis subject. (c). SRAD image with intimal contour of atherosclerosis subject.
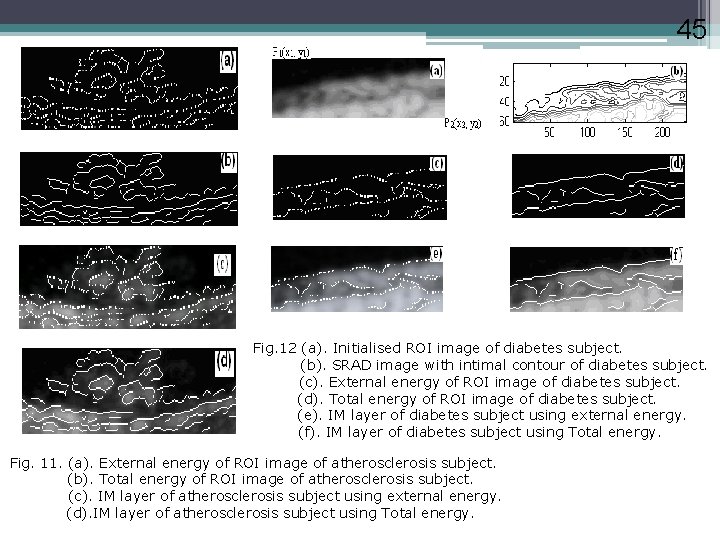
45 Fig. 12 (a). Initialised ROI image of diabetes subject. (b). SRAD image with intimal contour of diabetes subject. (c). External energy of ROI image of diabetes subject. (d). Total energy of ROI image of diabetes subject. (e). IM layer of diabetes subject using external energy. (f). IM layer of diabetes subject using Total energy. Fig. 11. (a). External energy of ROI image of atherosclerosis subject. (b). Total energy of ROI image of atherosclerosis subject. (c). IM layer of atherosclerosis subject using external energy. (d). IM layer of atherosclerosis subject using Total energy.
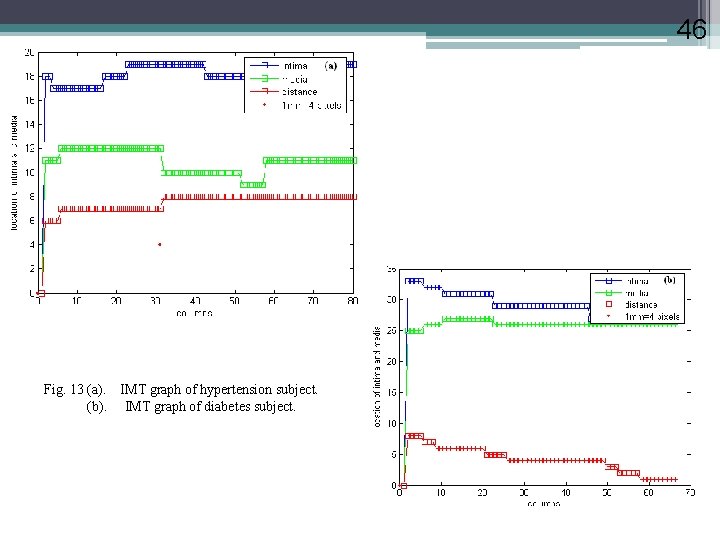
46 Fig. 13 (a). IMT graph of hypertension subject. (b). IMT graph of diabetes subject.
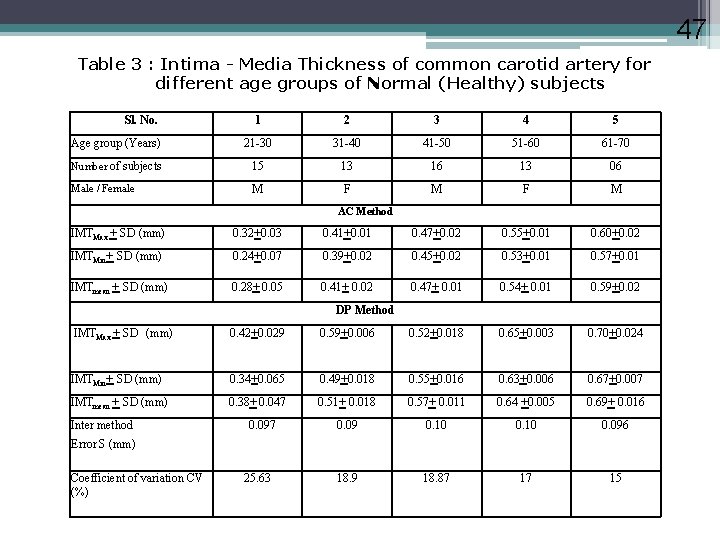
47 Table 3 : Intima - Media Thickness of common carotid artery for different age groups of Normal (Healthy) subjects Sl. No. 1 2 3 4 5 Age group (Years) 21 -30 31 -40 41 -50 51 -60 61 -70 Number of subjects 15 13 16 13 06 Male / Female M F M AC Method IMTMax + SD (mm) 0. 32+0. 03 0. 41+0. 01 0. 47+0. 02 0. 55+0. 01 0. 60+0. 02 IMTMin+ SD (mm) 0. 24+0. 07 0. 39+0. 02 0. 45+0. 02 0. 53+0. 01 0. 57+0. 01 IMTmean + SD (mm) 0. 28+ 0. 05 0. 41+ 0. 02 0. 47+ 0. 01 0. 54+ 0. 01 0. 59+0. 02 DP Method IMTMax + SD (mm) 0. 42+0. 029 0. 59+0. 006 0. 52+0. 018 0. 65+0. 003 0. 70+0. 024 IMTMin+ SD (mm) 0. 34+0. 065 0. 49+0. 018 0. 55+0. 016 0. 63+0. 006 0. 67+0. 007 IMTmean + SD (mm) 0. 38+ 0. 047 0. 51+ 0. 018 0. 57+ 0. 011 0. 64 +0. 005 0. 69+ 0. 016 0. 09 0. 10 0. 096 18. 9 18. 87 17 15 Inter method 0. 097 Error S (mm) Coefficient of variation CV (%) 25. 63
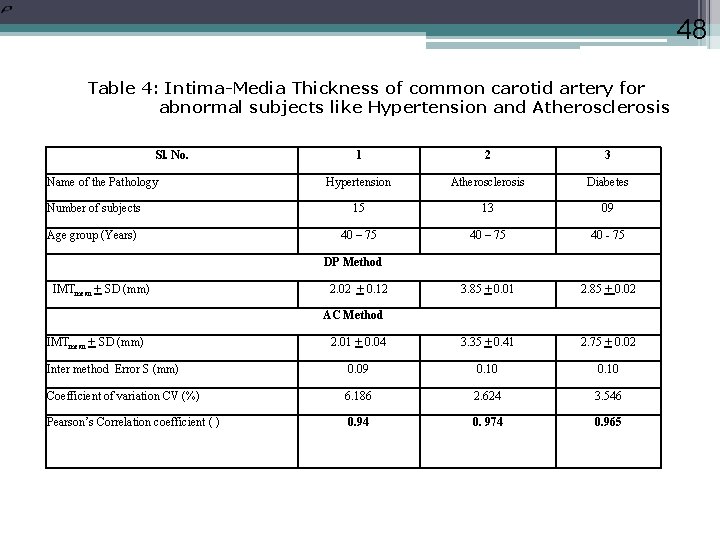
48 Table 4: Intima-Media Thickness of common carotid artery for abnormal subjects like Hypertension and Atherosclerosis Sl. No. 1 2 3 Hypertension Atherosclerosis Diabetes Number of subjects 15 13 09 Age group (Years) 40 – 75 40 - 75 3. 85 + 0. 01 2. 85 + 0. 02 2. 01 + 0. 04 3. 35 + 0. 41 2. 75 + 0. 02 Inter method Error S (mm) 0. 09 0. 10 Coefficient of variation CV (%) 6. 186 2. 624 3. 546 Pearson’s Correlation coefficient ( ) 0. 94 0. 974 0. 965 Name of the Pathology DP Method IMTmean + SD (mm) 2. 02 + 0. 12 AC Method IMTmean + SD (mm)
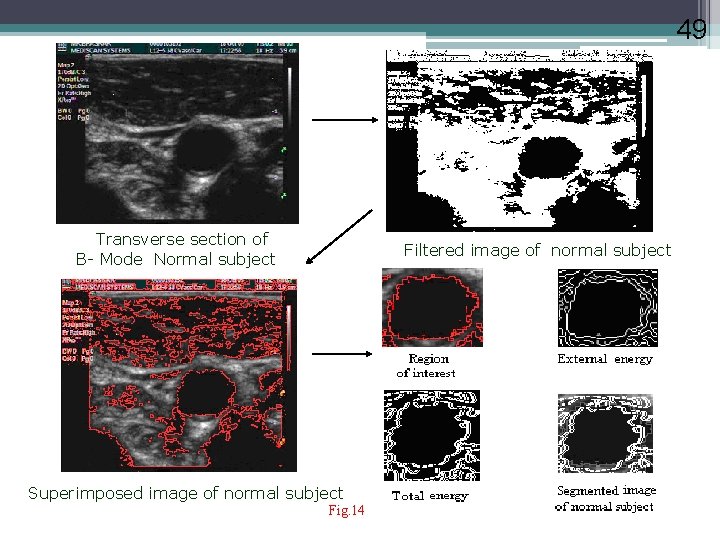
49 Transverse section of B- Mode Normal subject Filtered image of normal subject Superimposed image of normal subject Fig. 14 49
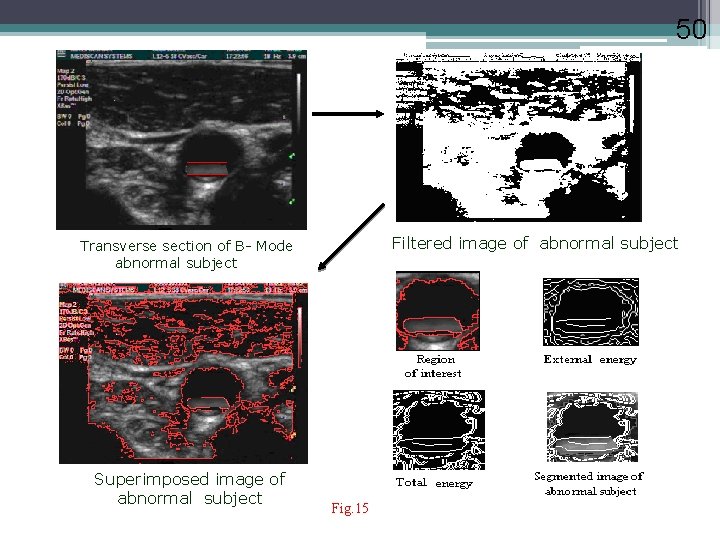
50 Filtered image of abnormal subject Transverse section of B- Mode abnormal subject Superimposed image of abnormal subject Fig. 15

51 Segmented Image of normal subject Fig. 16 Segmented Image of abnormal subject
52 Artificial Neural Networks Supervised method-Back-propagation algorithm 1 Collecting Data 2 Training The Ann 3 Testing The Ann
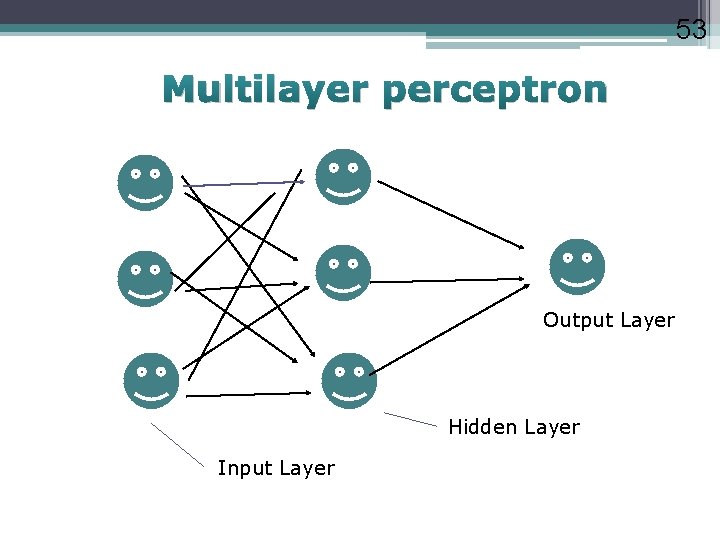
53 Multilayer perceptron Output Layer Hidden Layer Input Layer
![54 ANN PROGRAMMING Algorithm used [supervised method] BACK-PROPAGATION ALGORITHM based on steepest descent method 54 ANN PROGRAMMING Algorithm used [supervised method] BACK-PROPAGATION ALGORITHM based on steepest descent method](http://slidetodoc.com/presentation_image_h2/c1cdd200e5bda887d0af734e1988abd0/image-54.jpg)
54 ANN PROGRAMMING Algorithm used [supervised method] BACK-PROPAGATION ALGORITHM based on steepest descent method Advantages 1. Suited when input and output data are known. 2. Definitely converges to at least a local minima 3. Uses sigmoid function, i. e. , monotonically increasing
55 TRAINING PROCEDURE FOR ANN Given data - Training Data and Test Data. One hidden layer is used. 50 nodes are taken in the hidden layer. Decides sufficient degrees of freedom to learn the process correctly. Learning parameter used, =1. Momentum factor ( )=0. 01. Large variation in the input data can slow down or even prevent the training of the network. Hence all the inputs and outputs are scaled. continued. .
56 Only one node is decided in the output layer. The weight values are initialised with random weights. Errors in the hidden layer and output layer are calculated for backward propogation. Sigmoidal function between input and hidden layer and linear function between hidden and output layer. Performance function (MSE )is fixed as 0. 001. Weight values are adjusted according to error function until MSE is achieved. Weights are fixed after training.
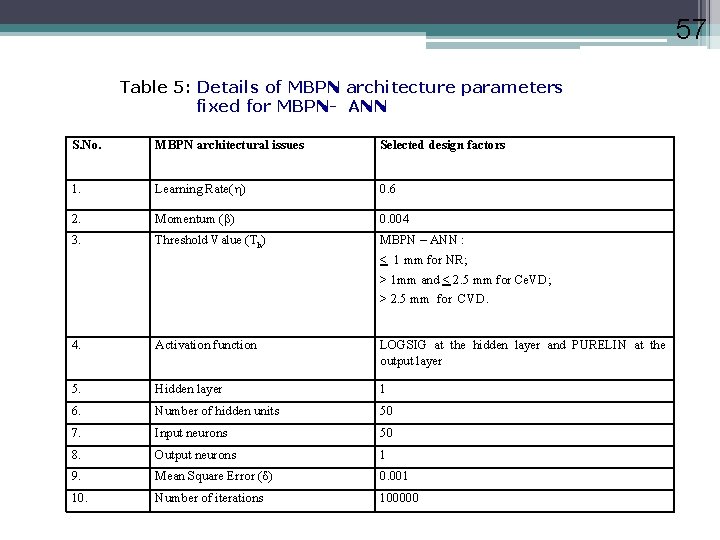
57 Table 5: Details of MBPN architecture parameters fixed for MBPN- ANN S. No. MBPN architectural issues Selected design factors 1. Learning Rate( ) 0. 6 2. Momentum ( ) 0. 004 3. Threshold Value (Th) MBPN – ANN : < 1 mm for NR; > 1 mm and < 2. 5 mm for Ce. VD; > 2. 5 mm for CVD. 4. Activation function LOGSIG at the hidden layer and PURELIN at the output layer 5. Hidden layer 1 6. Number of hidden units 50 7. Input neurons 50 8. Output neurons 1 9. Mean Square Error ( ) 0. 001 10. Number of iterations 100000
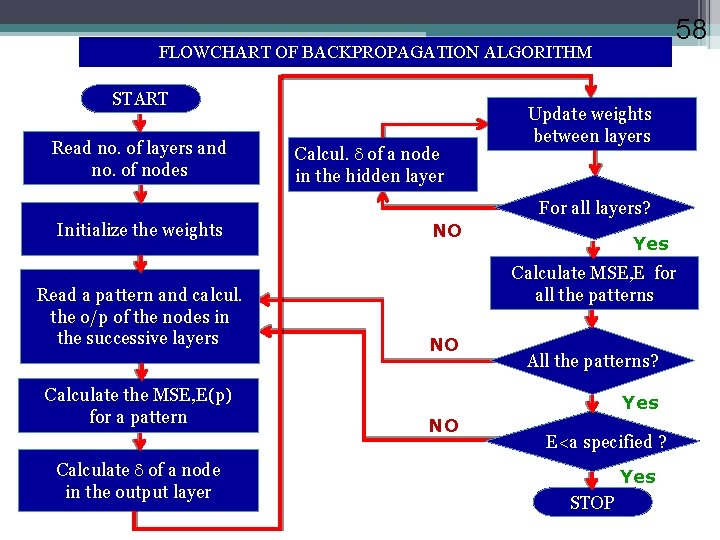
58 FLOWCHART OF BACKPROPAGATION ALGORITHM START Read no. of layers and no. of nodes Calcul. of a node in the hidden layer Update weights between layers For all layers? Initialize the weights Read a pattern and calcul. the o/p of the nodes in the successive layers Calculate the MSE, E(p) for a pattern Calculate of a node in the output layer NO Yes Calculate MSE, E for all the patterns NO All the patterns? Yes NO E<a specified ? Yes STOP

59 ü The output displayed by the test network is ‘Normal subject’ if the layer thickness values are less than 1 mm. ü If the thickness is more due to the pathologies developed or the thickness is more due to the abnormalities of the vessel, then the output displayed as ‘Abnormal subject’, such subject has to Meet the physicians for further diagnosis and treatment. ü Thus this study has recorded the outputs obtained for both the normal and abnormal subjects.
60 The CCA layers have been extracted using IDP technique and AC extraction technique. It was found from the results that the layer extraction using AC segmentation technique gives better results than IDP technique. It was also observed that the IMT linearly increases as age increases which may be due to the reduction in the circumference of the vascular tube. The estimated parameters can be used to early predict the blockage which is expected to cause the cerebrovascular and cardiovascular pathologies. The present study confirms that the AC technique can be successfully employed to reveal the early prediction of calcification or plaque in the subjects like hypertension and atherosclerosis. continued…
61 Decision support systems using neural network have been developed for classification of subjects. The categorization was done with MBPN module using extracted layer thickness values. It revealed that the optimized MBPN module offers appreciable classification efficiency, training and testing time. It was found that it facilitates to identify CCA category objectively. Such an automated process of identifying the category acts as a secondary observer and helps the medical experts in final decision making. The intermethod error was estimated as ± 0. 035 mm and ± 0. 09 mm for IDP and AC extraction techniques. continued…
62 It was also found that the coefficient of variation be 3. 55% and 18. 9% for IDP and AC extraction techniques. To be more specific, the AC technique provides better results as compared to IDP. The maximum classification efficiency of 96%, 90% and 92% was achieved with the learning rate = 0. 6 and momentum = 0. 004 for Normal (NR) images, Cerebrovascular Disease (Ce. VD) images and Cardiovascular Disease (CVD) images using MBPN. It is concluded that the soft computing techniques enhance the interpretation for better decision making. This work is done as a step towards the prevention of cerebrovascular and cardiovascular diseases, which are expected to be the cause of 20 million people death annually.

63 PUBLICATIONS N. Santhiyakumari, M. Madheswaran, (2006): ‘Estimation of layer thickness of arterio carotis using dynamic programming procedure’, Proceedings of 3 rd Cairo International Biomedical Engineering Conference, Cairo university, Cairo, IP 2 - 4, pp. 1 -4. N. Santhiyakumari, M. Madheswaran, (2008): ‘Non-Invasive evaluation of carotid artery wall thickness using improved dynamic programming technique’, Journal of Signal, Image and video processing, Springer International, Vol. 2, pp. 183 -193. N. Santhiyakumari, M. Madheswaran, (2007): ‘Extraction of Intimamedia layer of arteria- carotis and evaluation of its thickness using active contour approach’, Proceedings of International Conference on intelligent and advanced systems, KL convention centre, Kula Lumpur, Malaysia, IP_MS 1, pp. 1 -5.
![64 [4]. N. Santhiyakumari, M. Madheswaran, (2008): ‘Automatic detection of the intima and media 64 [4]. N. Santhiyakumari, M. Madheswaran, (2008): ‘Automatic detection of the intima and media](http://slidetodoc.com/presentation_image_h2/c1cdd200e5bda887d0af734e1988abd0/image-64.jpg)
64 [4]. N. Santhiyakumari, M. Madheswaran, (2008): ‘Automatic detection of the intima and media layers of common carotid artery using active contour segmentation’, Applied Soft Computing, Elsevier journal, Manuscript ID ASOC-D-08 -00051, under review. [5]. N. Santhiyakumari, M. Madheswaran, (2008): ‘Analysis of atherosclerosis for identification of cerebrovascular and cardiovascular diseases using active contour segmentation of carotid artery’, Proceedings of International Symposium on Global Trends in Biomedical Informatics Research, Education and Commercialization, Chennai, Vol 1, pp. 40. [6]. N. Santhiyakumari, M. Madheswaran, (2008): ‘Analysis of atherosclerosis for identification of cerebrovascular and cardiovascular diseases using active contour segmentation of carotid artery’, International Journal of Medical Engineering and Informatics, Manuscript No. IJBECHI-0308 -22, Accepted.

65 REFERENCES 1. Jegelevicius D. and Lukoševicius A. (2002), ‘Ultrasonic measurements of human carotid artery wall intima-media Thickness’, Ultragarsas, pp. 43 -47. 2. Pierre-Jean Touboul (2002), ‘Clinical impact of intima media measurement’, European Journal of Ultrasound’, Vol 16, pp. 105 -/113. 3. Sheng-Fang Huang, Ruey-Feng Chang, Dar-Ren Chen and Woo Kyung Moon (2004), ‘Characterization of Speculation on Ultrasound Lesions’, IEEE trans. on Medical Imaging, Vol. 23, pp. 111 -121. 4. Loizou C. P. , Christodoulou. C. , Pattischis C. S. , Istepanian R. S. H. , Pantziaris M. and Nicolaides A. (2002), ‘Speckle Reduction in Ultrasound Images of Atherosclerotic Carotid Plaque’, Proc. 14 th IEEE Intl. Conf. on Digital Signal Processing, Santorini, Greece, pp. 525 -528. continued. . .

66 Queries ? THANK YOU…!
- Slides: 66