Implementation of fully automated specimen processing and data
- Slides: 1

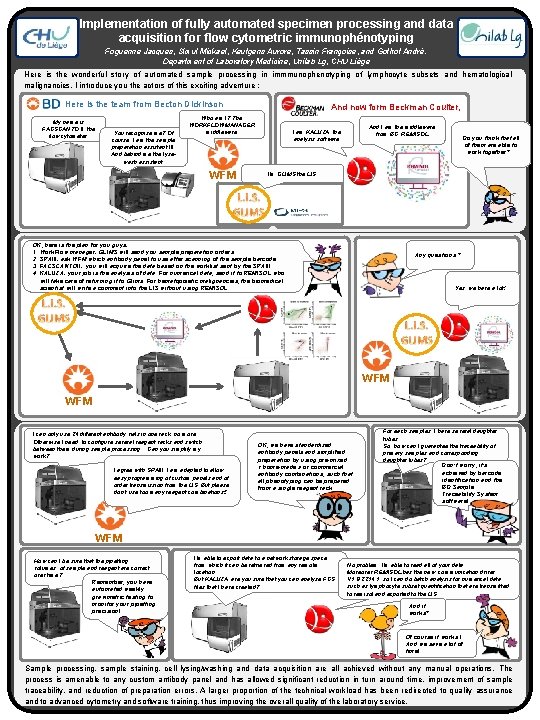
Implementation of fully automated specimen processing and data acquisition for flow cytometric immunophénotyping Foguenne Jacques, Simul Mickael, Keutgens Aurore, Tassin Françoise, and Gothot André. Department of Laboratory Medicine, Unilab Lg, CHU Liège Here is the wonderful story of automated sample processing in immmunophenotyping of lymphocyte subsets and hematological malignancies. I introduce you the actors of this exciting adventure : Here is the team from Becton Dickinson My name is FACSCANTO II, the flow cytometer. You recognise me? Of course, I am the sample preparation assistant III. And behind me the lysewash assistant. And now form Beckman Coulter, Who am I? The WORKFLOWMANAGER middleware WFM And I am the middleware from BC, REMISOL I am KALUZA, the analysis software Do you think that all of them are able to work together? . . . I’m GLIMS the LIS. OK, here is the plan for you guys: 1. Work. Flow manager: GLIMS will send you sample preparation orders. 2. SPAIII: ask WFM which antibody panel to use after scanning of the sample barcode. 3. FACSCANTOII: you will acquire the data based on the worklist sent by the SPAIII 4. KALUZA: your job is the analysis of data. For numerical data, send it to REMISOL who will take care of returning it to Glims. For hematopoietic malignancies, the biomedical scientist will write a comment into the LIS without using REMISOL. Any questions ? Yes, we have a lot! R R WFM I can only use 24 different antibody vials in one rack, no more. Otherwise I need to configure several reagent racks and switch between them during sample processing. . . Can you simplify my work? I agree with SPAIII, I am adapted to allow easy programming of custom panels and of we have a lot! order transmission from. Yes, the LIS. But please R don’t use too many reagent combinations!! OK, we have standardized antibody panels and simplified preparation by using pre-mixed « home-made » or commercial antibody combinations, such that all phenotyping can be prepared from a single reagent rack. For each samples, I have several daughter tubes. So, how can I guarantee the traceability of primary samples and corresponding daughter tubes? Don’t worry, it’s achieved by barcode identification and the BD Sample Traceability System software! R WFM How can I be sure that the pipetting volumes of sample and reagent are correct over time? Remember, you have automated weekly gravimetric testing to monitor your pipetting precision! R I’m able to export data to a network storage space from which it can be retrieved from any remote location. But KALUZA, are you sure that you can analyse FCS files that I have created? No problem, I’m able to read all of your data. Moreover, REMISOL has the new communication driver V 1. 8. 2214. 1, so I can do batch analysis for numerical data such as lymphocyte subset quantification that are transmitted to remisol and exported to the LIS. Rc And it works? Of course it works ! And we save a lot of time! Sample processing, sample staining, cell lysing/washing and data acquisition are all achieved without any manual operations. The process is amenable to any custom antibody panel and has allowed significant reduction in turn around time, improvement of sample traceability, and reduction of preparation errors. A larger proportion of the technical workload has been redirected to quality assurance and to advanced cytometry and software training, thus improving the overall quality of the laboratory service.