Impact of protein detergent interactions on NMR structure



- Slides: 17

Impact of protein – detergent interactions on NMR structure determination Linda Columbus Department of Chemistry University of Virginia

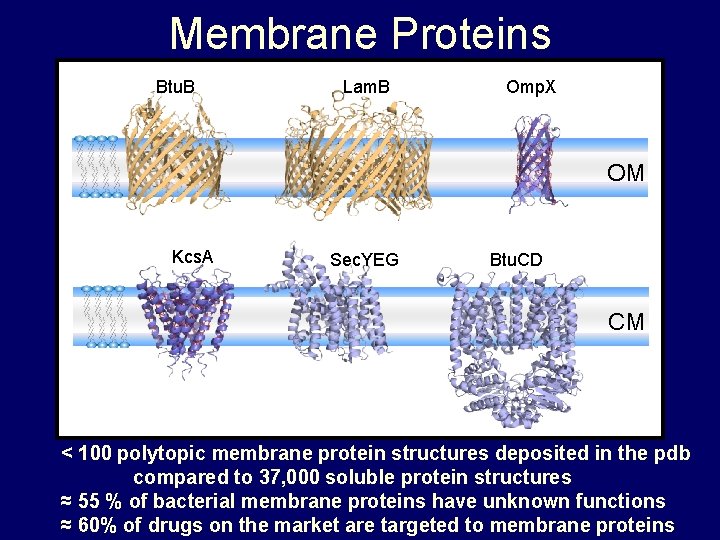
Membrane Proteins Btu. B Lam. B Omp. X OM Kcs. A Sec. YEG Btu. CD CM < 100 polytopic membrane protein structures deposited in the pdb compared to 37, 000 soluble protein structures ≈ 55 % of bacterial membrane proteins have unknown functions ≈ 60% of drugs on the market are targeted to membrane proteins

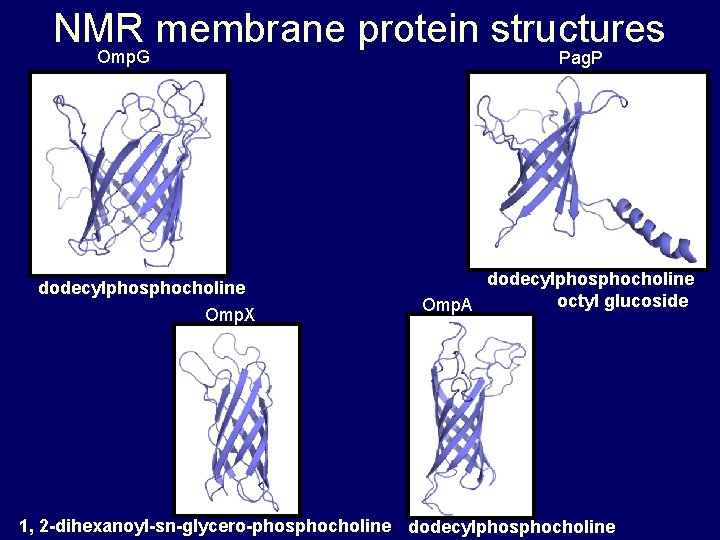
NMR membrane protein structures Omp. G Pag. P dodecylphosphocholine Omp. X dodecylphosphocholine octyl glucoside Omp. A 1, 2 -dihexanoyl-sn-glycero-phosphocholine dodecylphosphocholine

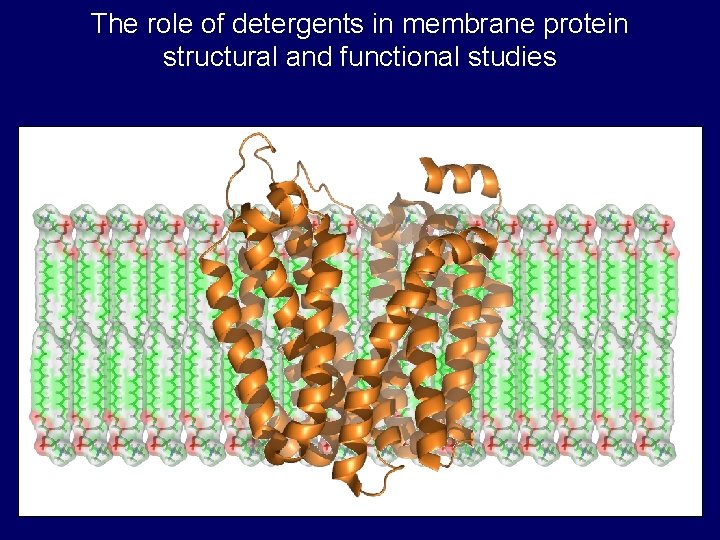
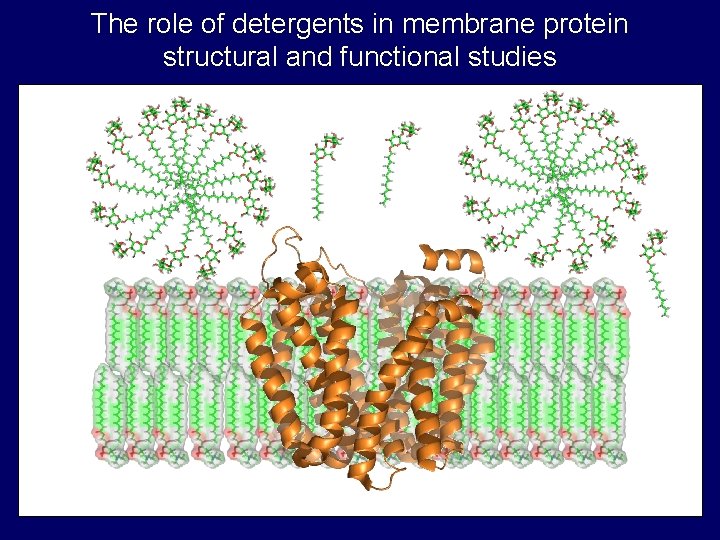
The role of detergents in membrane protein structural and functional studies

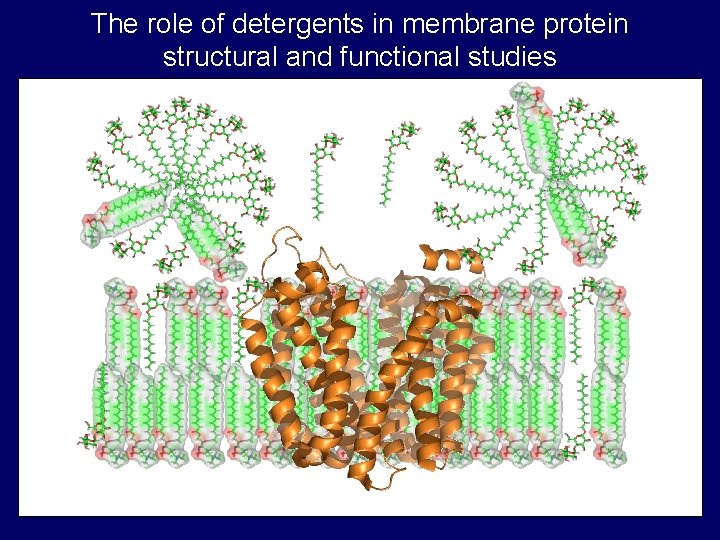
The role of detergents in membrane protein structural and functional studies

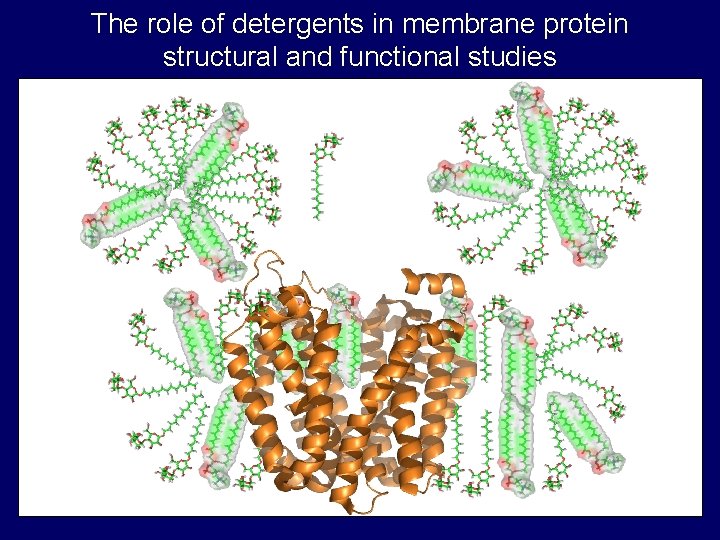
The role of detergents in membrane protein structural and functional studies

The role of detergents in membrane protein structural and functional studies

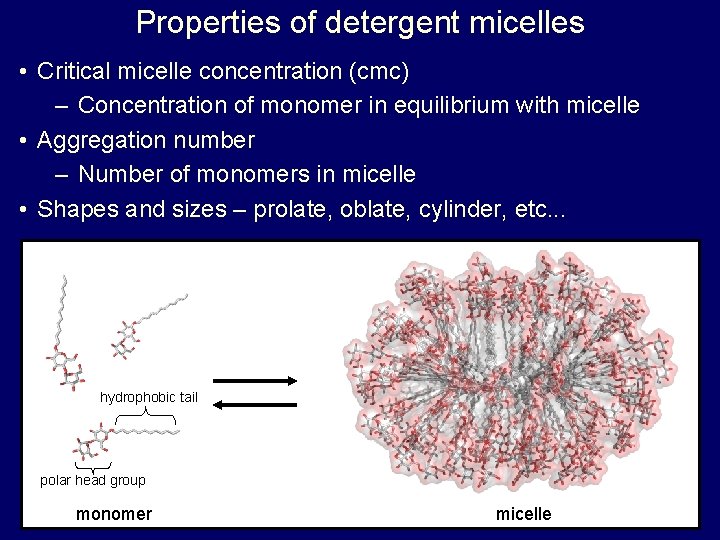
Properties of detergent micelles • Critical micelle concentration (cmc) – Concentration of monomer in equilibrium with micelle • Aggregation number – Number of monomers in micelle • Shapes and sizes – prolate, oblate, cylinder, etc. . . hydrophobic tail polar head group monomer micelle

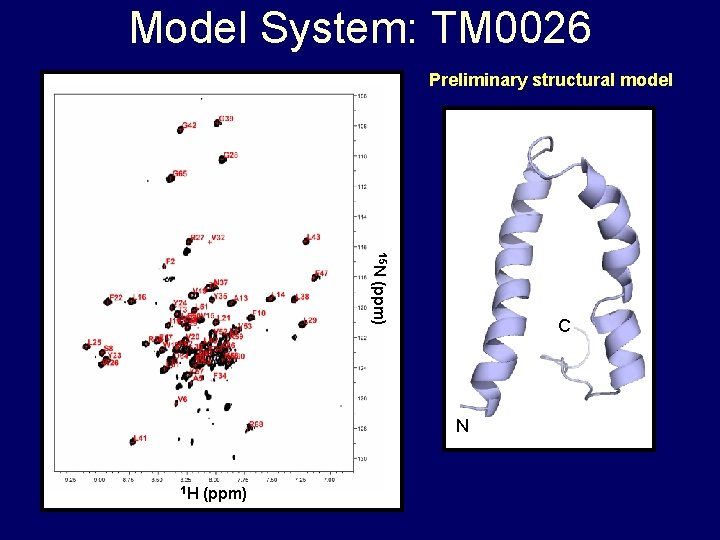
Model System: TM 0026 Preliminary structural model 15 N (ppm) C N 1 H (ppm)

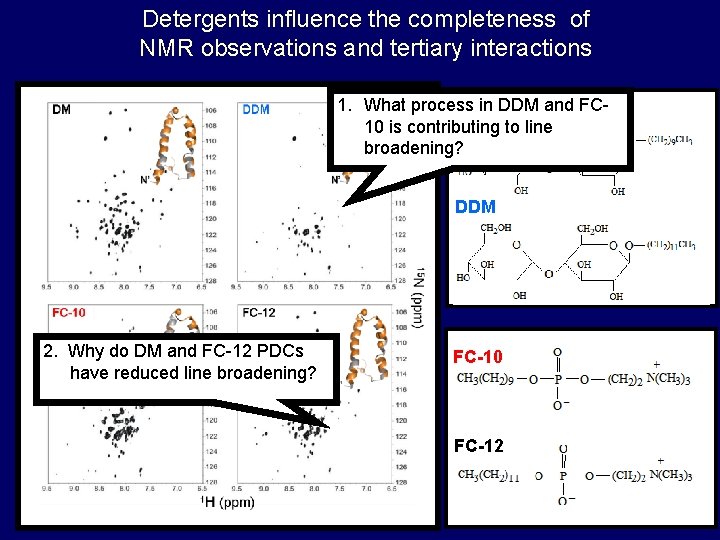
Detergents influence the completeness of NMR observations and tertiary interactions 1. What process DMin DDM and FC 10 is contributing to line broadening? DDM 2. Why do DM and FC-12 PDCs have reduced line broadening? FC-10 FC-12

What happens in FC-10 and DDM that contributes to line broadening?

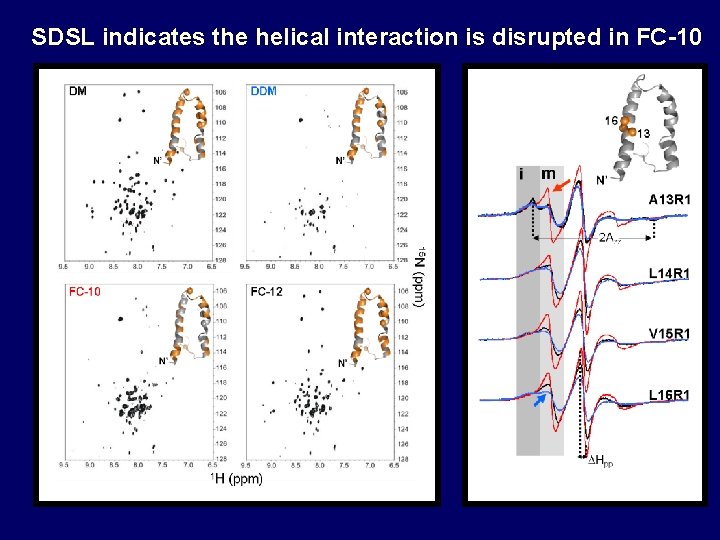
SDSL indicates the helical interaction is disrupted in FC-10

Why do DM and FC-12 give rise to quality NMR spectra?

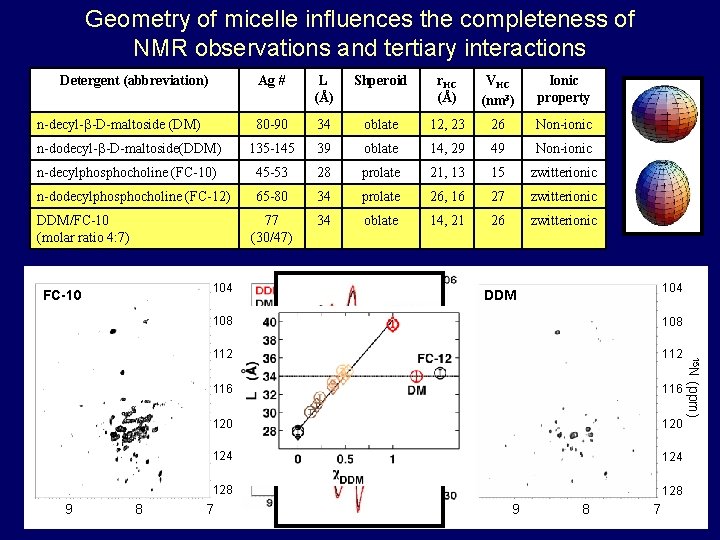
Geometry of micelle influences the completeness of NMR observations and tertiary interactions Detergent (abbreviation) Ag ## Ag L (Å) Shperoid r. HC (Å) VHC (nm 3) Ionic property 80 -90 34 oblate 12, 23 26 Non-ionic n-dodecyl- -D-maltoside (DDM) n-dodecyl- -D-maltoside(DDM) 135 -145 39 oblate 14, 29 49 Non-ionic n-decylphosphocholine (FC-10) 45 -53 28 prolate 21, 13 15 zwitterionic n-dodecylphosphocholine (FC-12) 65 -80 34 34 prolate 26, 16 16 27 27 zwitterionic 77 (30/47) 34 oblate 14, 21 26 zwitterionic n-decyl- -D-maltoside (DM) DDM/FC-10 (molar ratio 4: 7) 104 FC-10 108 112 116 120 124 128 7 1 H (ppm) 9 8 7 (ppm) 8 108 15 N 9 104 DDM

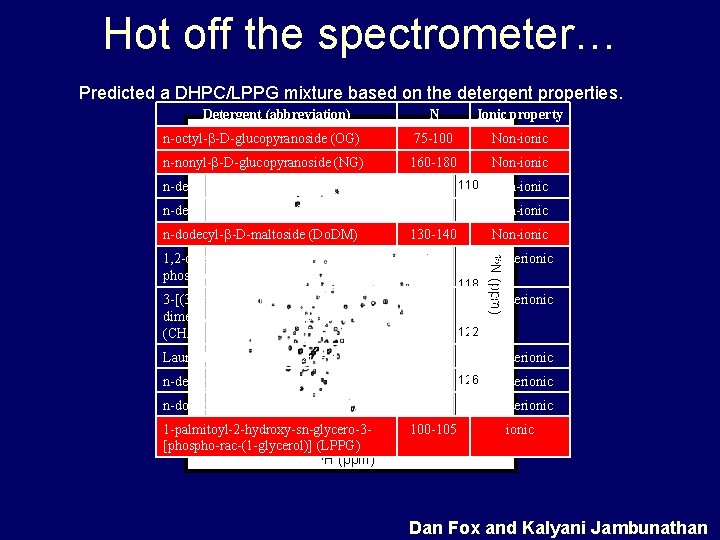
Hot off the spectrometer… Predicted a DHPC/LPPG mixture based on the detergent properties. Detergent (abbreviation) N Ionic property n-octyl- -D-glucopyranoside (OG) 75 -100 106 Non-ionic n-nonyl- -D-glucopyranoside (NG) 160 -180 n-decyl- -D-glucopyranoside (DG) ND n-decyl- -D-maltoside (DM) n-dodecyl- -D-maltoside (Do. DM) 110 70 -75 Non-ionic 130 -140 114 Non-ionic 3 -[(3 -cholamidopropyl)dimethylammonio]-1 -propane sulfonate (CHAPS) 14 -18 zwitterionic 118 (ppm) 35 -40 15 N 1, 2 -dihexanoyl-sn-glycerophosphocholine (DHPC) Lauryldimethylyamine oxide (LDAO) Non-ionic zwitterionic 122 NC zwitterionic n-decylphosphocholine (FC-10) 35 -45 126 zwitterionic n-dodecylphosphocholine (FC-12) 65 -70 zwitterionic 100 -105 7 ionic 1 -palmitoyl-2 -hydroxy-sn-glycero-39 8 [phospho-rac-(1 -glycerol)] (LPPG) 1 H (ppm) Dan Fox and Kalyani Jambunathan

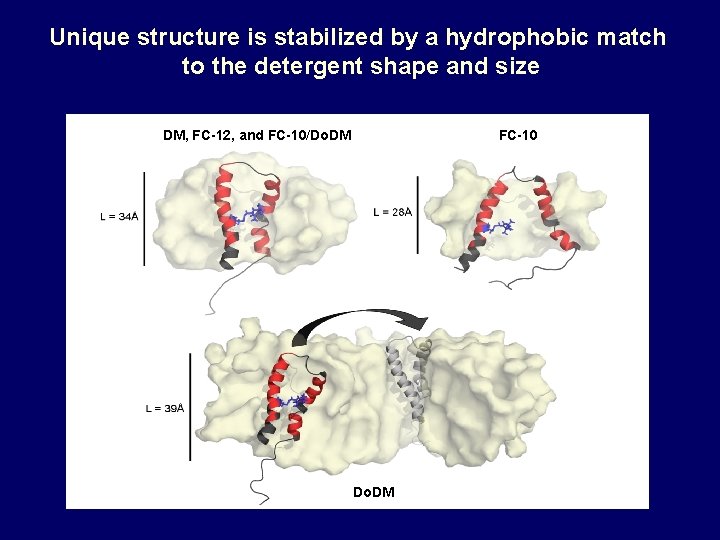
Unique structure is stabilized by a hydrophobic match to the detergent shape and size DM, FC-12, and FC-10/Do. DM FC-10 Do. DM

Acknowledgements Small Angle X-ray Scattering Scott Lesley Jan Lipfert Gerard Kroon Bernhard Geierstanger Sebastian Doniach Kalyani Jambunathan Iza Bielnicka NRSA 1 F 32 GM 068286 UVA Start-up funds Bill Peairs Thien Nguyen Brett Kroncke Dan Fox Chris Reyes Justin Kim