Immunology Biol 465 Minot State University Spring 2020

Immunology Biol 465 Minot State University Spring 2020 MWF 9 -9: 50 am Lab Tuesdays 9 am-noon

Lecture 11 February 12 th , 2020 New corona virus has a name. COVID-19 What did you read about bats as its “reservoir”. – What is a virus reservoir? – Why do bats carry so many viruses? – From an immunological view (based on stuff we’ve recently learned, hypothesize on why they don’t they get sick/die from COVID-19 and other viruses.

Where we? Induced innate immunity Complement activation and effector functions. You should be able to to tell me: – How complement can be activated (pathways) – How complement works to kill bacteria or aid in inducing more innate mechanisms – How complement proteins are activated at the molecular level – How complement activation is controlled NK cell responses to viruses---how does it work?
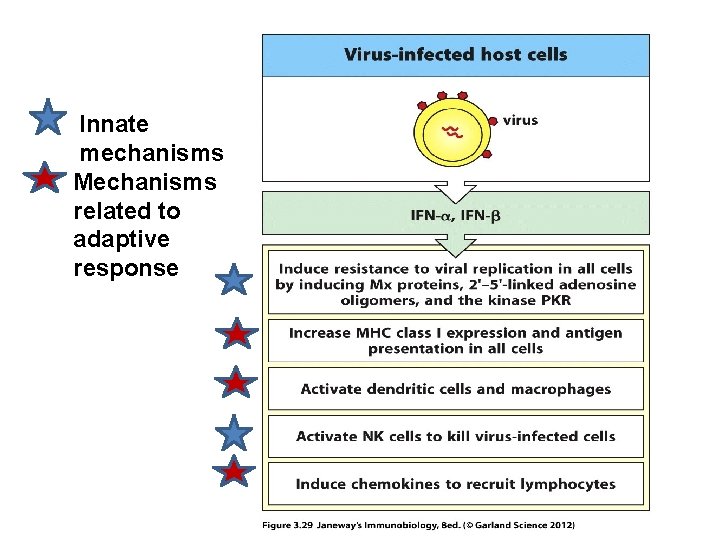
Innate mechanisms Mechanisms related to adaptive response
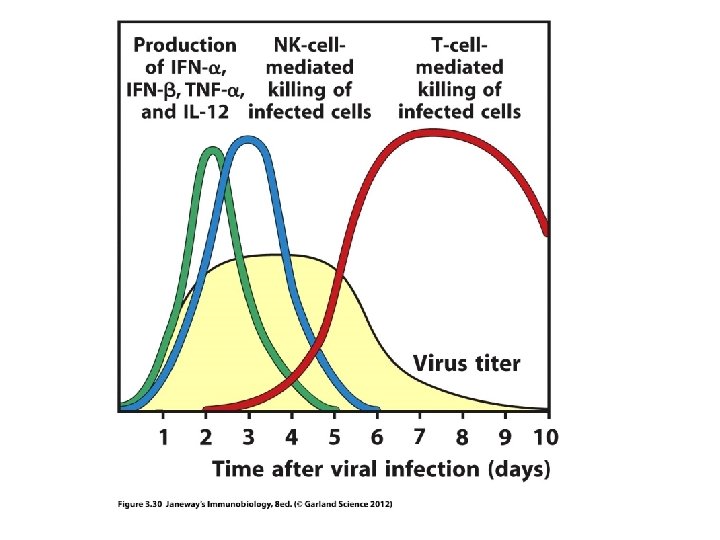

Moving on…to adaptive immunity • Let’s review some basic differences in antigen recognition in innate and adaptive immunity • Think of the differences in PAMP receptors and lymphocyte antigen receptors
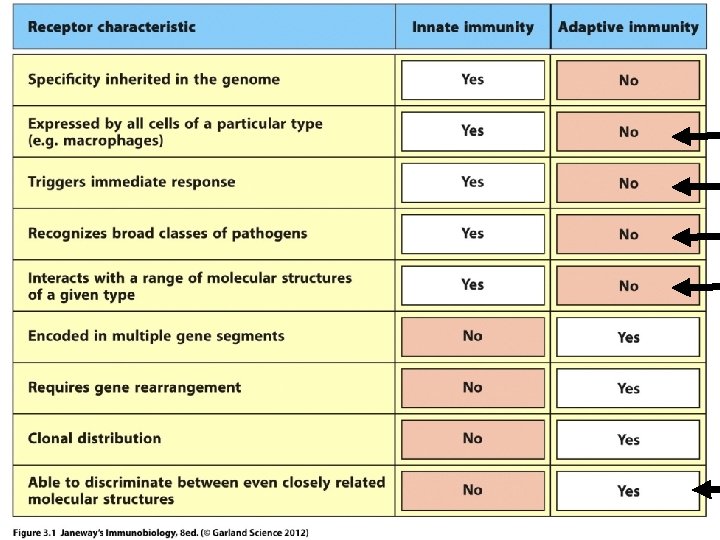
Moving on… We’ll begin thinking about adaptive immunity. Chapter 3 introduces B and T-cells and their antigen receptors

• Practice putting these concepts (with arrows) into a sentence using your own words.
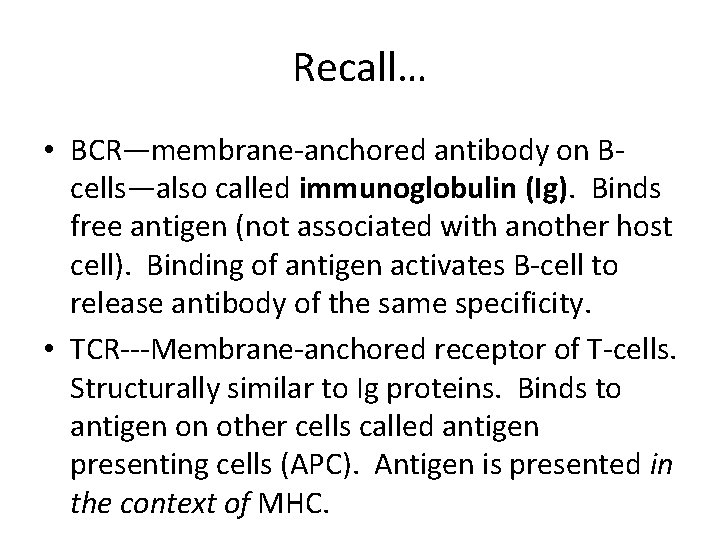
Recall… • BCR—membrane-anchored antibody on Bcells—also called immunoglobulin (Ig). Binds free antigen (not associated with another host cell). Binding of antigen activates B-cell to release antibody of the same specificity. • TCR---Membrane-anchored receptor of T-cells. Structurally similar to Ig proteins. Binds to antigen on other cells called antigen presenting cells (APC). Antigen is presented in the context of MHC.
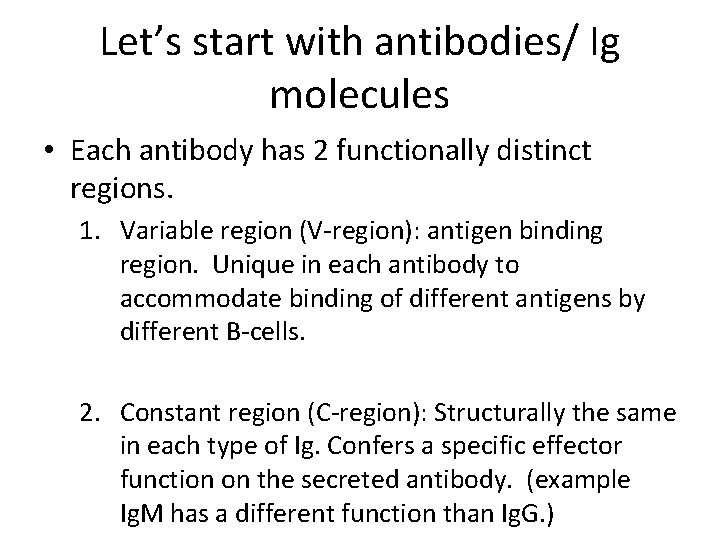
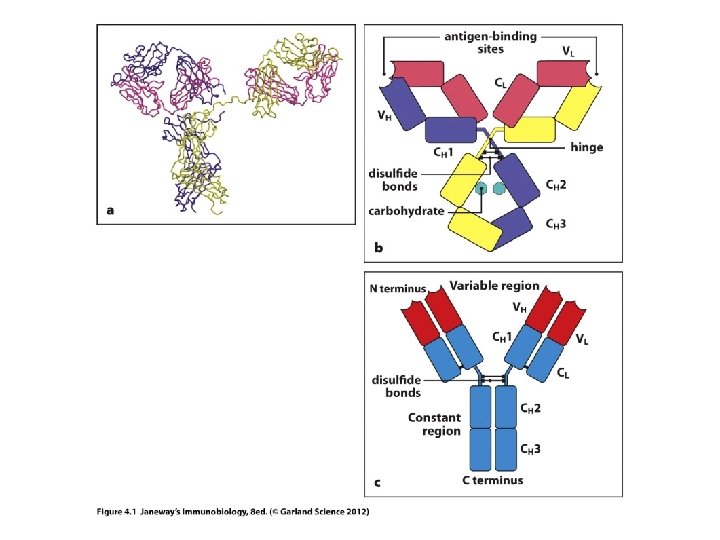
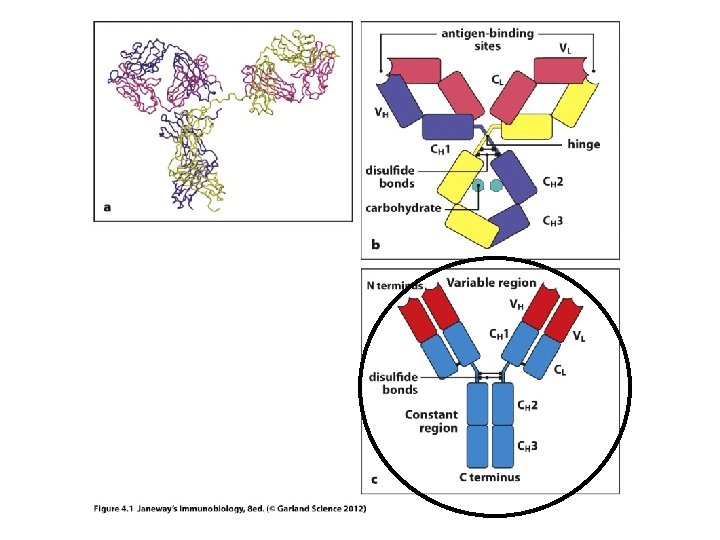
Let’s start with antibodies/ Ig molecules • Each antibody has 2 functionally distinct regions. 1. Variable region (V-region): antigen binding region. Unique in each antibody to accommodate binding of different antigens by different B-cells. 2. Constant region (C-region): Structurally the same in each type of Ig. Confers a specific effector function on the secreted antibody. (example Ig. M has a different function than Ig. G. )
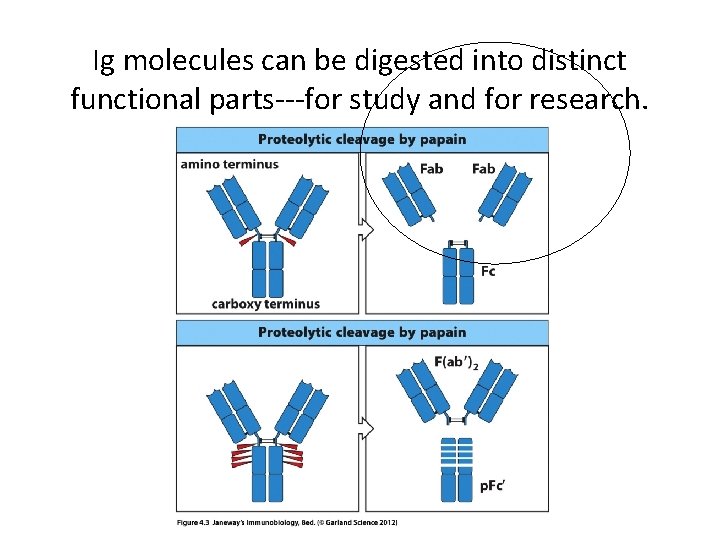
Ig molecules can be digested into distinct functional parts---for study and for research.

• • Recall what antibodies do… Neutralize Opsonize Activate complement
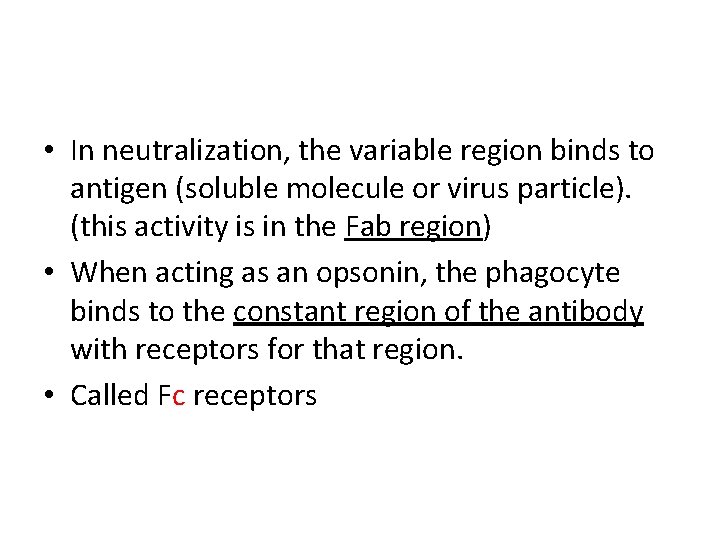
• In neutralization, the variable region binds to antigen (soluble molecule or virus particle). (this activity is in the Fab region) • When acting as an opsonin, the phagocyte binds to the constant region of the antibody with receptors for that region. • Called Fc receptors
Ig types (Isotypes) • 5 classes of Ig, each with a different effector function. • Ig. A • Ig. D • Ig. E • Ig. G • Ig. M (They differ in the Fc region—and in their effector function)

We will learn more about each type…. • Ig. M and Ig. D: are the types that remain anchored to B-cell membrane • Ig. M is first type to be secreted • Ig. G most abundant in serum
• In the activation and maturation of a B-cell, antibodies with the same variable region can obtain new constant regions by a DNA recombination event in the Ig gene. • This is known as Isotype switch. For example one B-cell starts making Ig. M and usually switches to another isotype, such as Ig. G
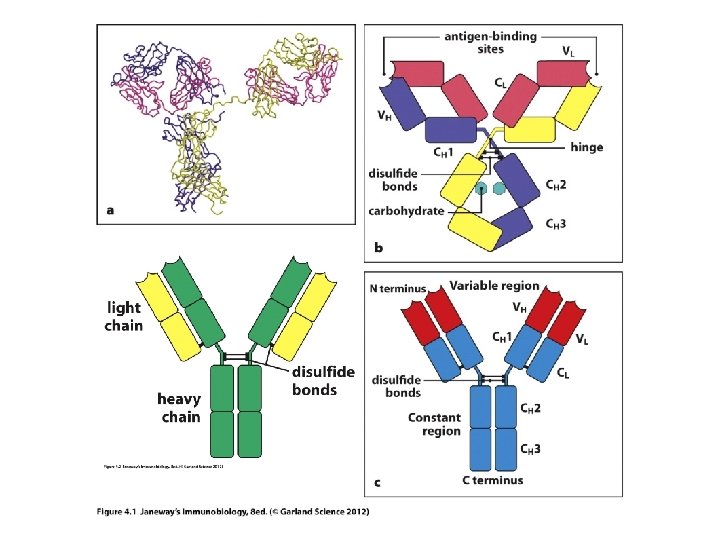
• In order to understand expression of Ig (and TCR) you may have to review proteins and protein synthesis. —Brief overview on board. • https: //www. youtube. com/watch? v=OEWOZS _JTgk (Includes ideas of “induced” gene expression---think of cytokine activity. • Antibodies are multi-subunit proteins. Made from 2 large and 2 small proteins. In antibody lingo, the large are referred to as heavy chains and the small as light chains.
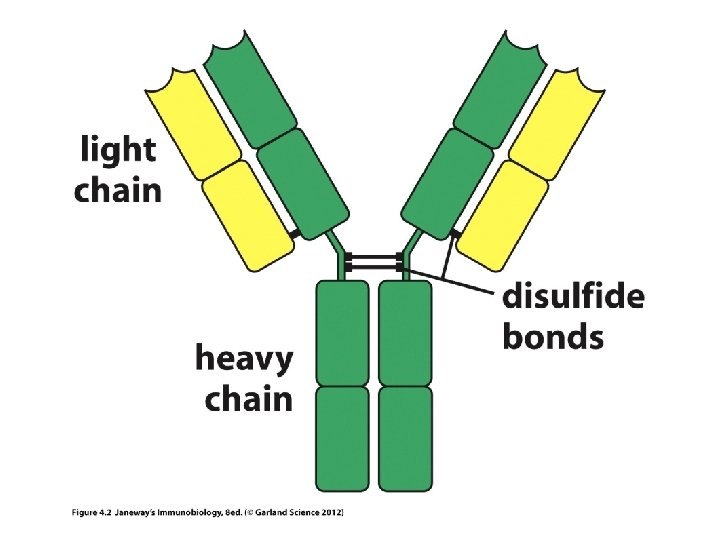

• Chain simply refers to a chain of amino acids, a protein. • Any given antibody will have 2 identical light chains and 2 identical heavy chains
• Each chain in an Ig molecule is encoded by a different gene. – Humans have a singe H-chain gene – Humans have 2 different L-chain genes (lambda l and kappa k)
Holding Ig molecules together • Disulfide bonds can hold proteins together. The amino acids, methionine and cysteine have sulfur and can form S-S bonds. – The 2 H-chains are anchored to each other via S-S bonds – Each light chain is linked to one H-chain via an S-S bond.
• Recall, each heavy and light chain has a variable region and a constant region. • How is each put together? (They are one continuous protein, encoded by a single gene), but the gene itself is put together in a very unusual way—no other genes are assembled like this.

Let’s review protein structure first… • N-terminus • C-terminus Levels of structure Primary Secondary Tertiary Quaternary
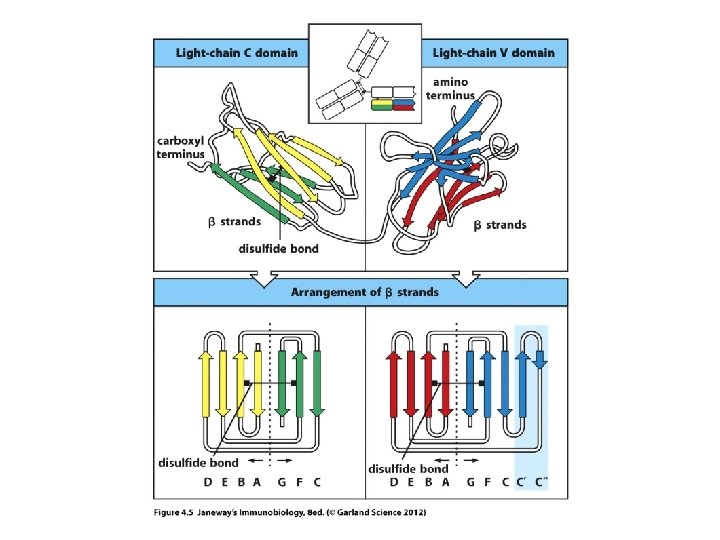
• The models show the predominant secondary structure is b-sheet. Antibodies are made of proteins that fold in a predictable way---a number of proteins fold this way and are included in the Ig superfamily (TCR proteins fold like this too. ) They fold into Ig-like domains.

• Observe carefully…each chain heavy and light, contributes to both the V-region and to the Cregion. • If you think of the primary structure of each protein, the N-terminal 110 amino acids contribute to the V-region.
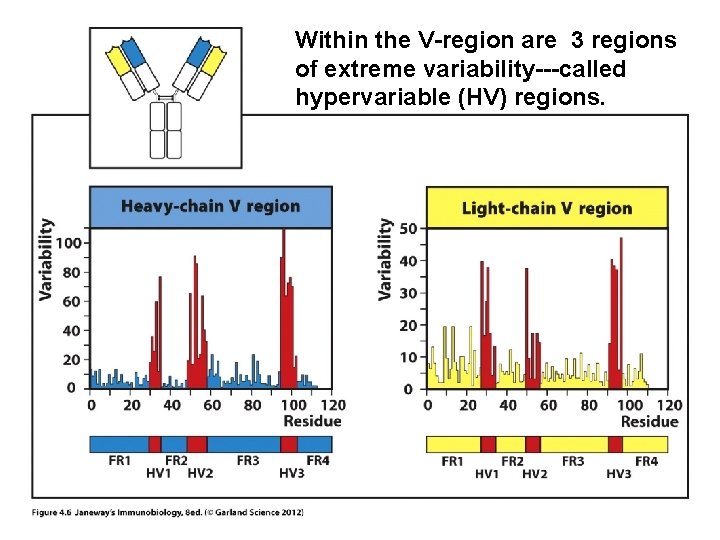
Within the V-region are 3 regions of extreme variability---called hypervariable (HV) regions.
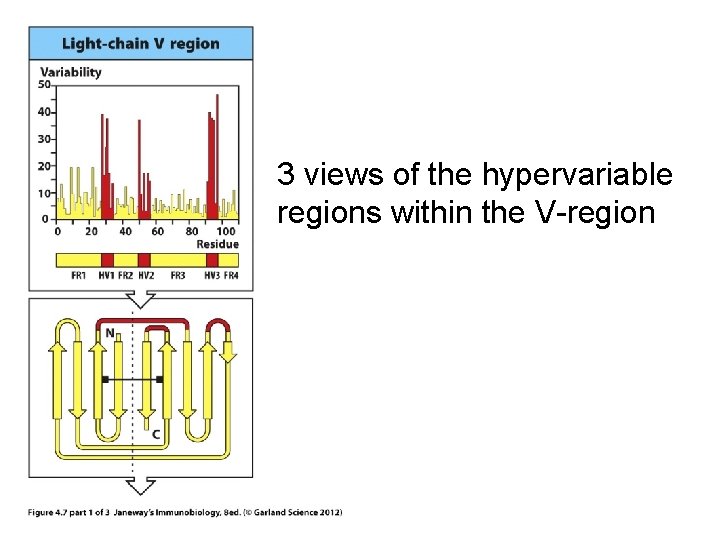
3 views of the hypervariable regions within the V-region
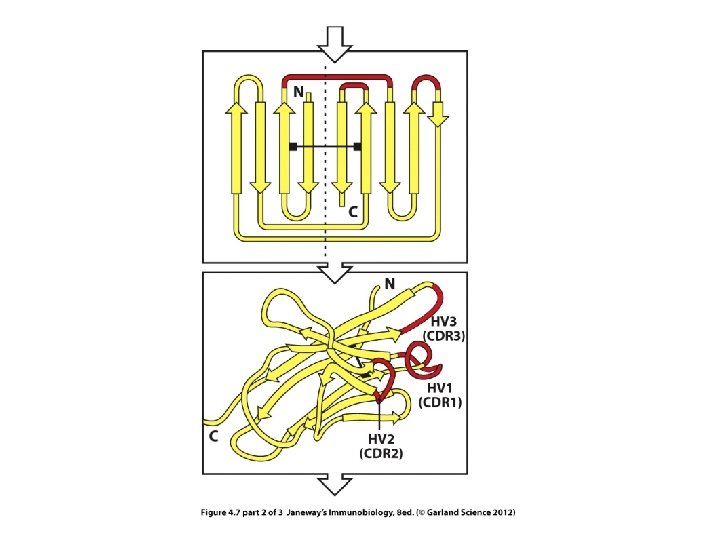
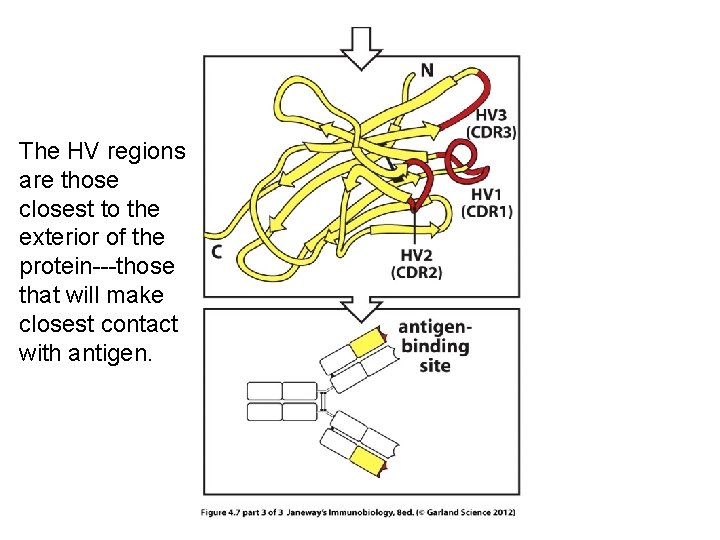
The HV regions are those closest to the exterior of the protein---those that will make closest contact with antigen.
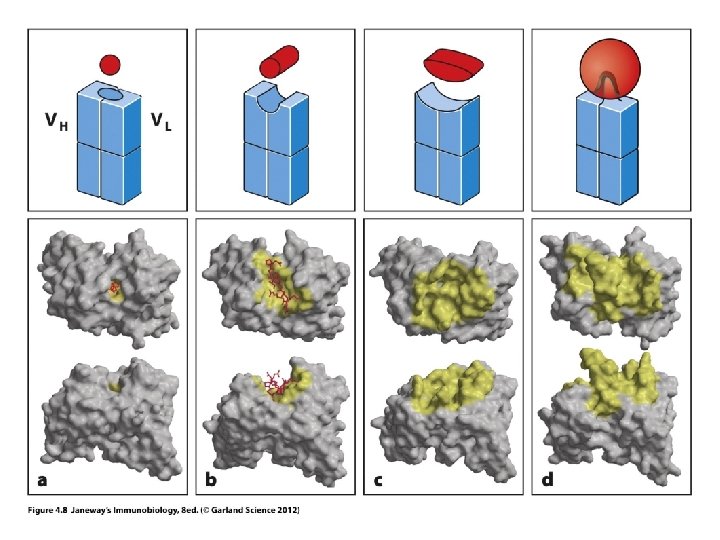

Please review • How DNA encodes m. RNA (decoding via transcription) • How m. RNA is translated into an amino acid sequence • How different amino acid sequences fold into different shapes and thus interact with different
- Slides: 35