Immunoglobulin Definition Immunoglobulins are a group of large

Immunoglobulin
Definition • Immunoglobulins are a group of large glycoproteins either function as antibodies or are structural similar to antibodies. • Ig ≠ Ab

Two existence forms of Ig • Soluble Abs • Membrane bound immune molecules
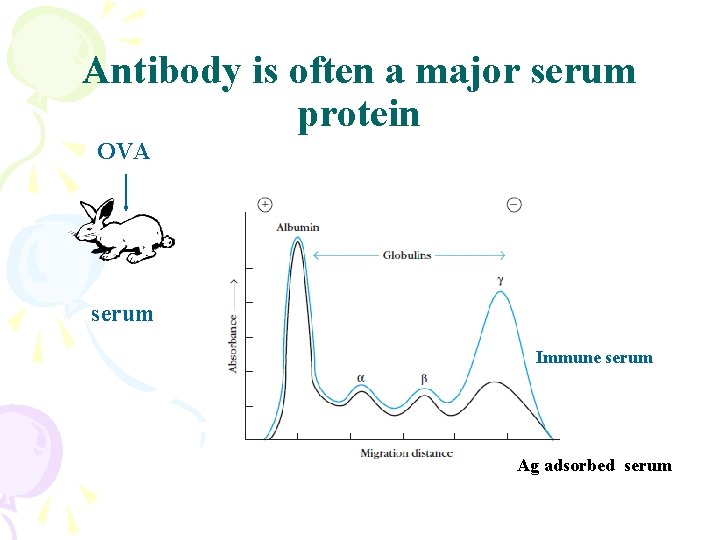
Antibody is often a major serum protein OVA serum Immune serum Ag adsorbed serum
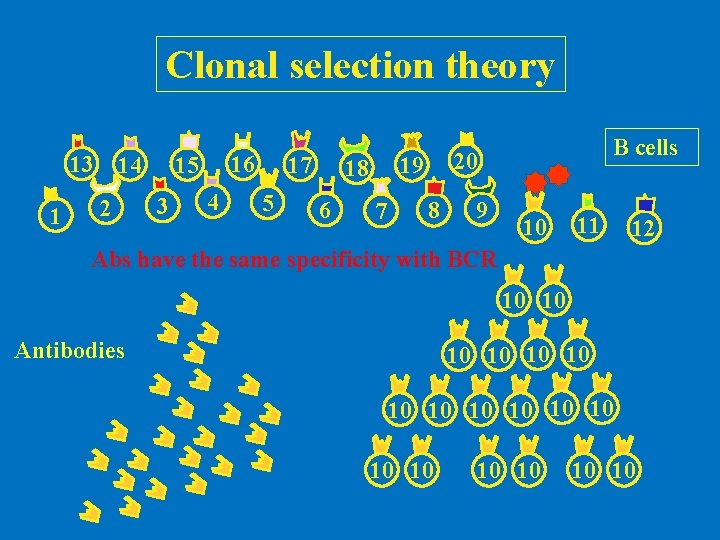
Clonal selection theory 13 1 14 2 16 15 3 4 17 5 6 20 19 18 7 B cells 8 9 10 11 12 Abs have the same specificity with BCR 10 10 Antibodies 10 10 10 10
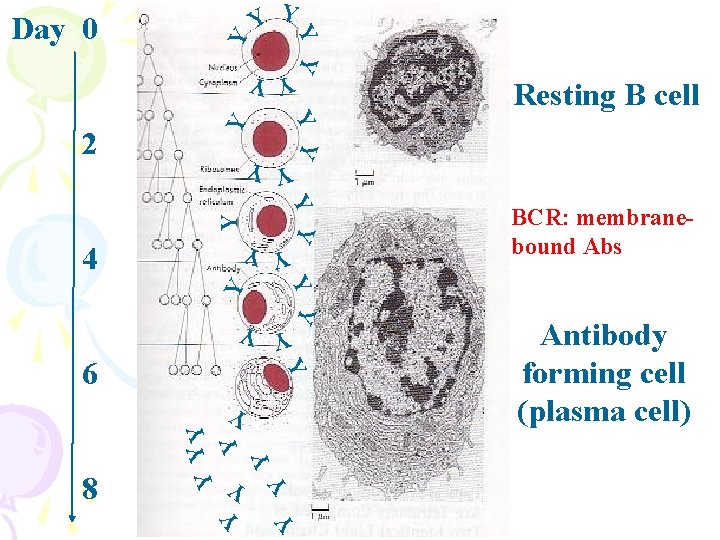
Y YY 4 Y YY YY Y 6 Y YY Y Y Y 8 Resting B cell YY 2 YY Day 0 Y Y BCR: membranebound Abs Antibody forming cell (plasma cell)

Two questions 1. How does the antibody system manage to be so specific? 2. After combined specifically with Ags, how can Abs protect us?
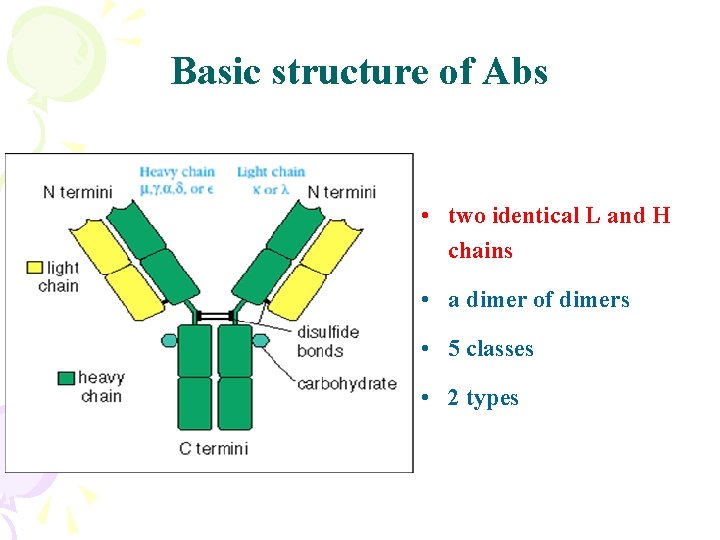
Basic structure of Abs • two identical L and H chains • a dimer of dimers • 5 classes • 2 types
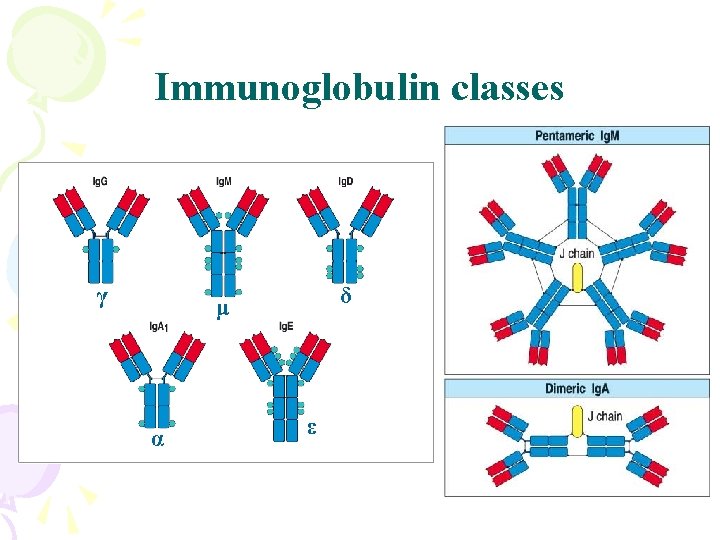
Immunoglobulin classes γ δ μ α ε
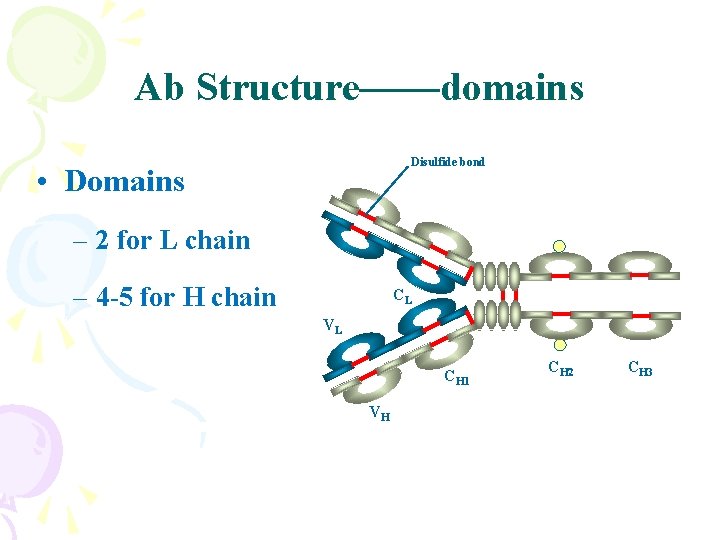
Ab Structure——domains Disulfide bond • Domains – 2 for L chain – 4 -5 for H chain CL VL CH 1 VH CH 2 CH 3
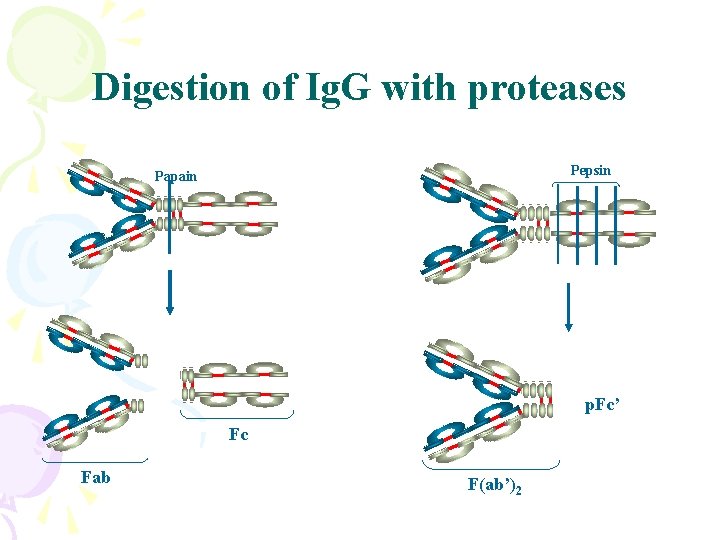
Digestion of Ig. G with proteases Pepsin Papain p. Fc’ Fc Fab F(ab’)2
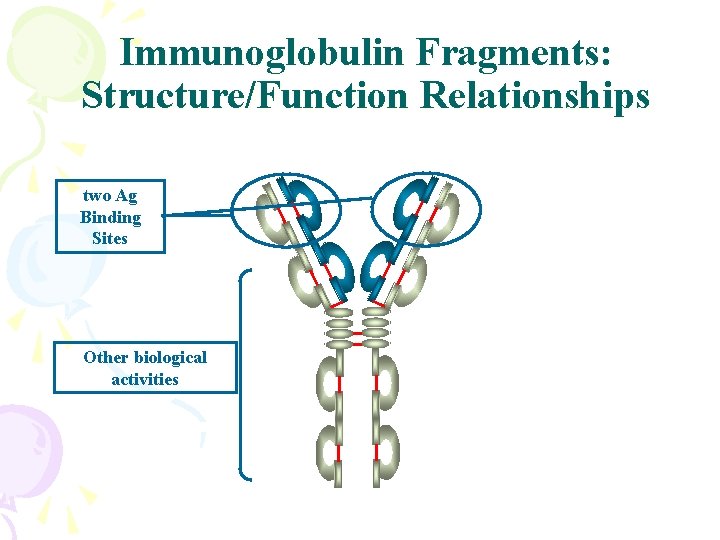
Immunoglobulin Fragments: Structure/Function Relationships two Ag Binding Sites Other biological activities
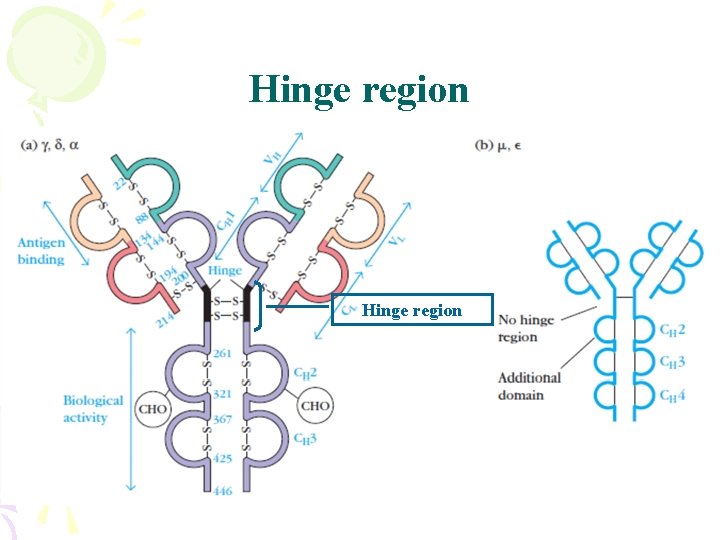
Hinge region

Two questions 1. Among Fab, Fc, and the hinge region, which determine the specificity of antibody? 2. What is the structural basis for Fab to distinguish subtle differences between different antigens?
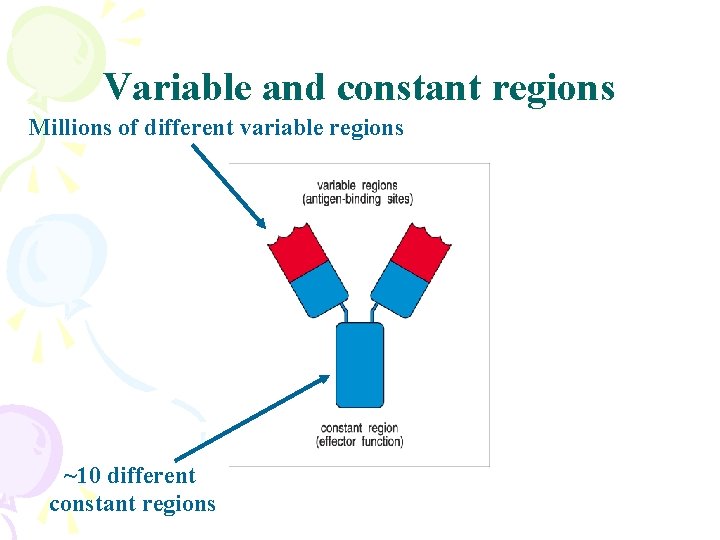
Variable and constant regions Millions of different variable regions ~10 different constant regions
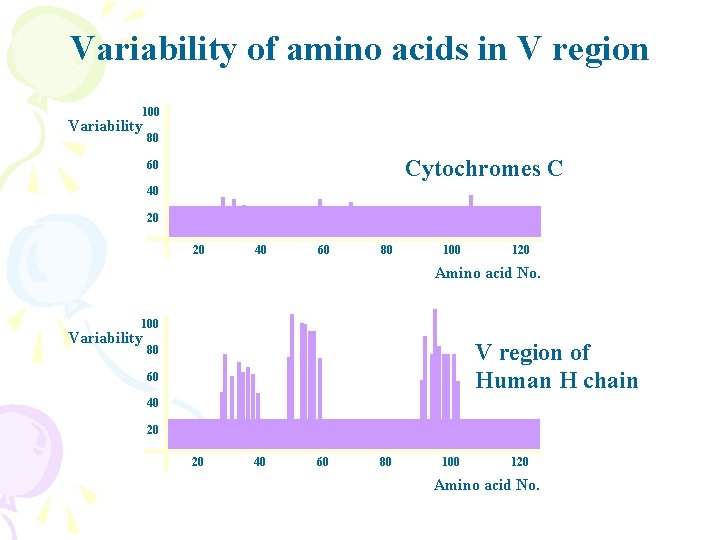
Variability of amino acids in V region 100 Variability 80 Cytochromes C 60 40 20 20 40 60 80 100 120 Amino acid No. 100 Variability V region of Human H chain 80 60 40 20 20 40 60 80 100 120 Amino acid No.
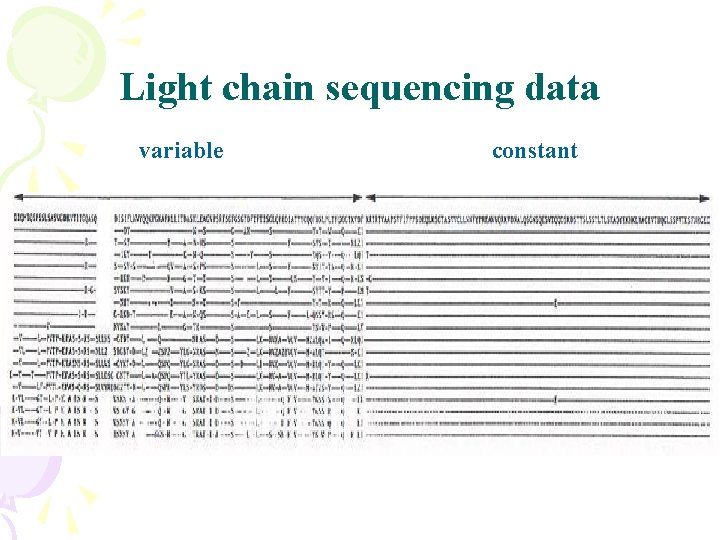
Light chain sequencing data variable constant
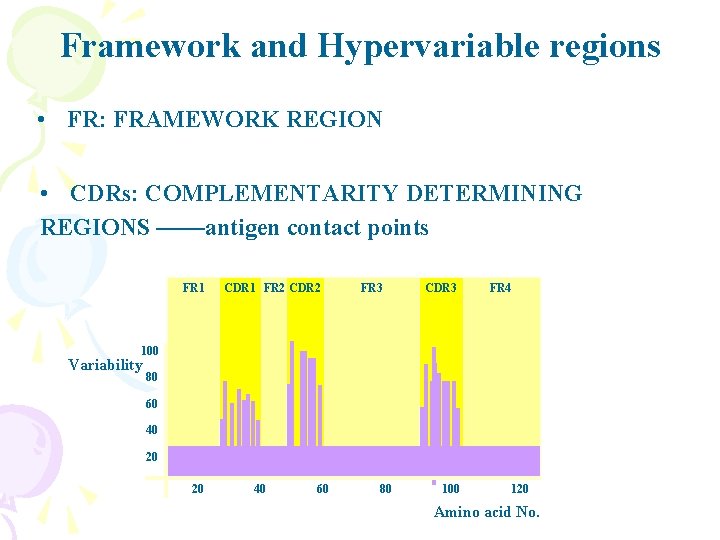
Framework and Hypervariable regions • FR: FRAMEWORK REGION • CDRs: COMPLEMENTARITY DETERMINING REGIONS ——antigen contact points FR 1 CDR 1 FR 2 CDR 2 FR 3 CDR 3 FR 4 100 Variability 80 60 40 20 20 40 60 80 100 120 Amino acid No.
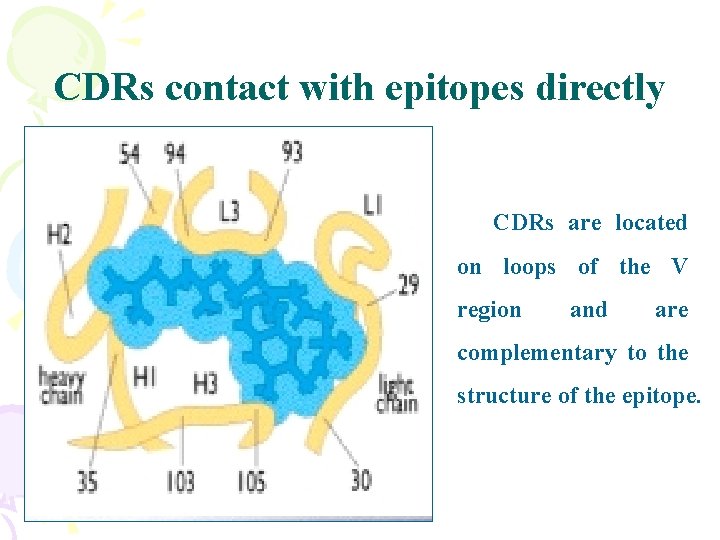
CDRs contact with epitopes directly CDRs are located on loops of the V region and are complementary to the structure of the epitope.
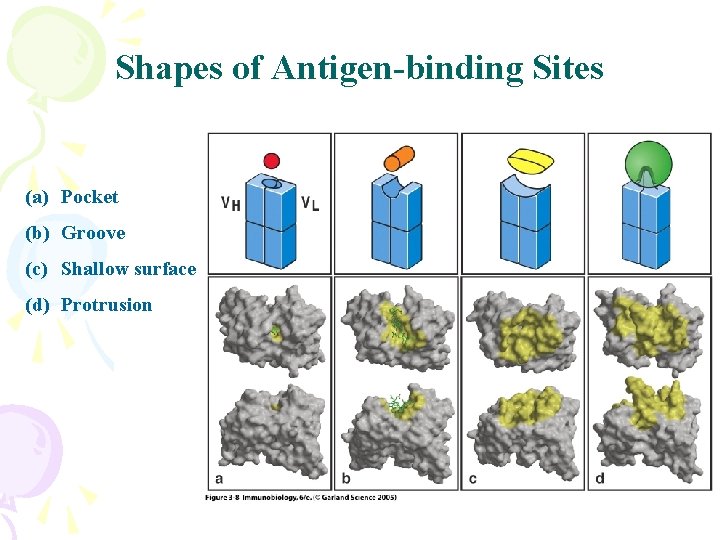
Shapes of Antigen-binding Sites (a) Pocket (b) Groove (c) Shallow surface (d) Protrusion
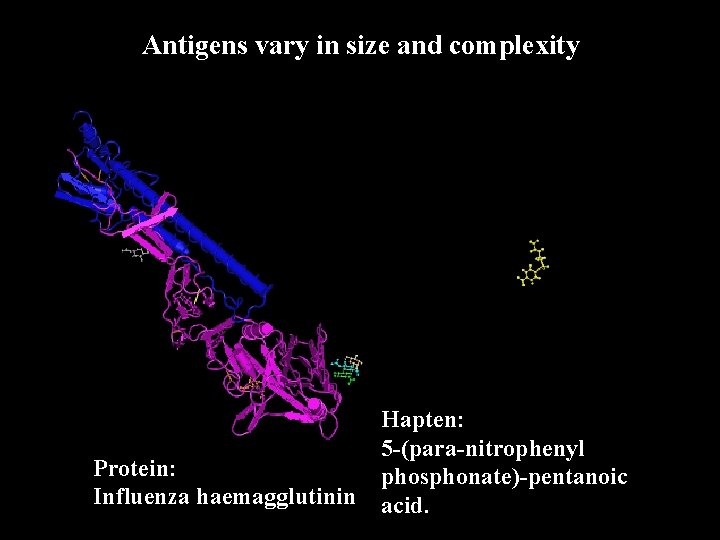
Antigens vary in size and complexity Protein: Influenza haemagglutinin Hapten: 5 -(para-nitrophenyl phosphonate)-pentanoic acid.
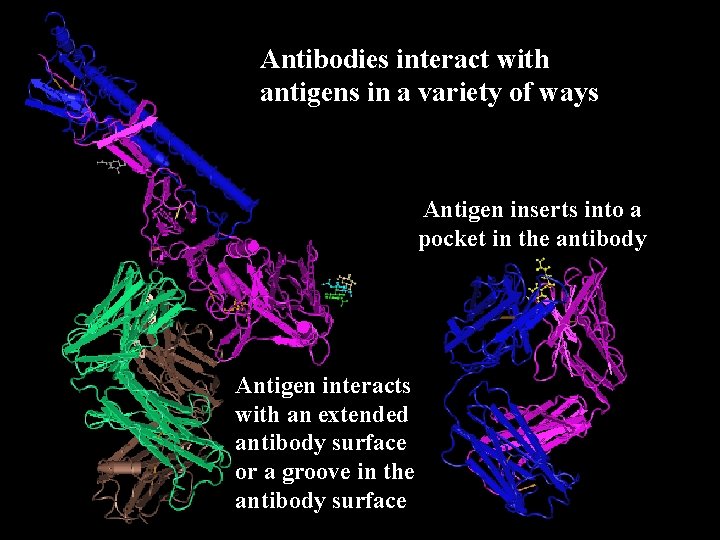
Antibodies interact with antigens in a variety of ways Antigen inserts into a pocket in the antibody Antigen interacts with an extended antibody surface or a groove in the antibody surface
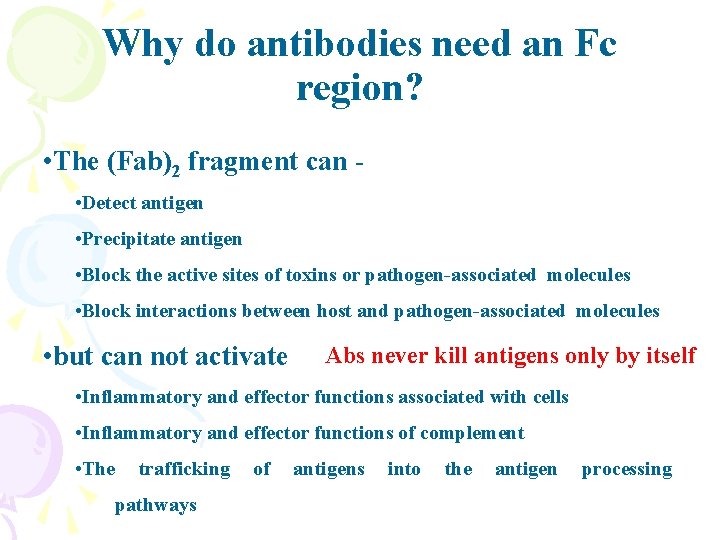
Why do antibodies need an Fc region? • The (Fab)2 fragment can • Detect antigen • Precipitate antigen • Block the active sites of toxins or pathogen-associated molecules • Block interactions between host and pathogen-associated molecules • but can not activate Abs never kill antigens only by itself • Inflammatory and effector functions associated with cells • Inflammatory and effector functions of complement • The trafficking pathways of antigens into the antigen processing
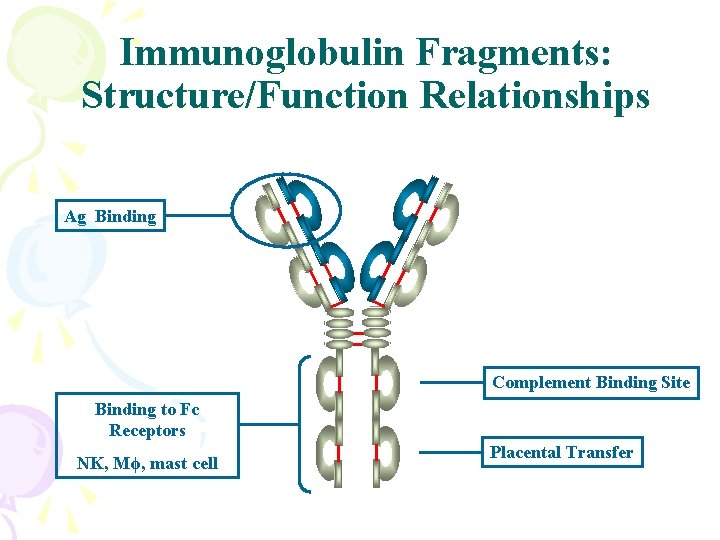
Immunoglobulin Fragments: Structure/Function Relationships Ag Binding Complement Binding Site Binding to Fc Receptors NK, Mϕ, mast cell Placental Transfer
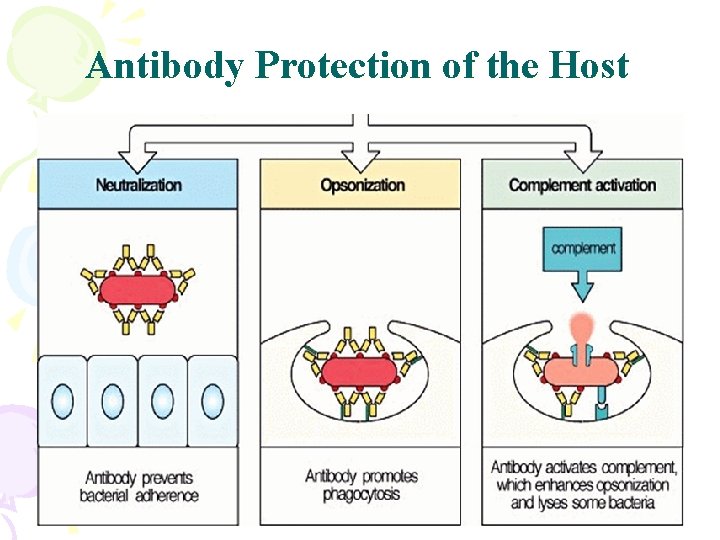
Antibody Protection of the Host
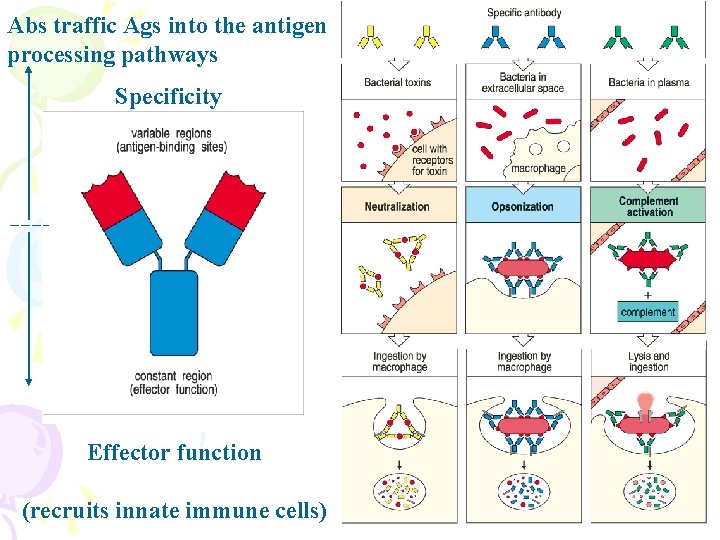
Abs traffic Ags into the antigen processing pathways Specificity Effector function (recruits innate immune cells)
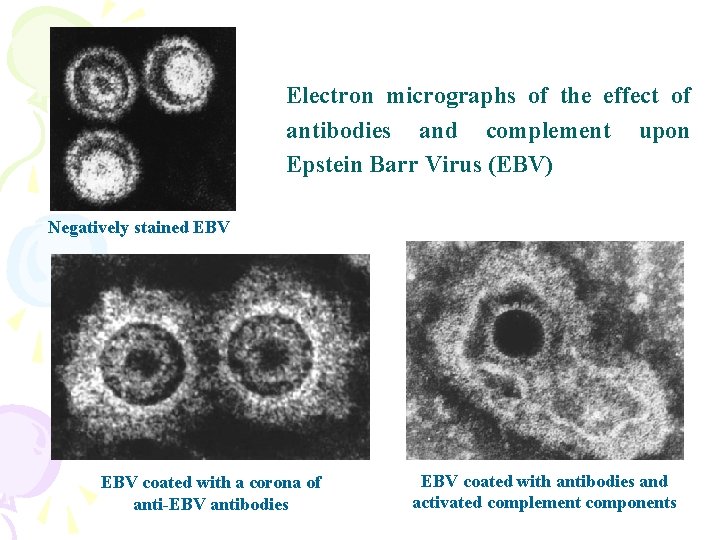
Electron micrographs of the effect of antibodies and complement upon Epstein Barr Virus (EBV) Negatively stained EBV coated with a corona of anti-EBV antibodies EBV coated with antibodies and activated complement components
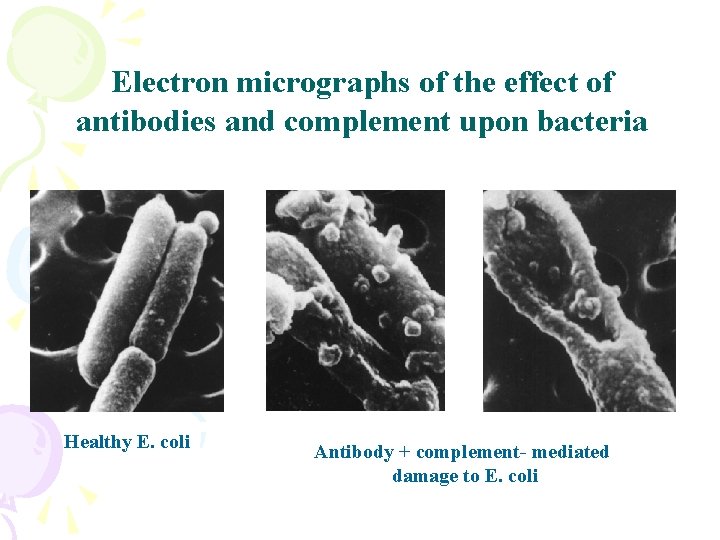
Electron micrographs of the effect of antibodies and complement upon bacteria Healthy E. coli Antibody + complement- mediated damage to E. coli
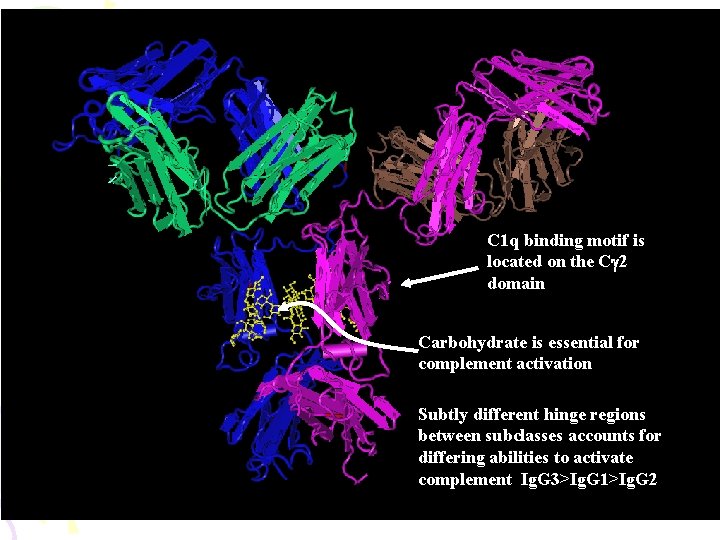
C 1 q binding motif is located on the Cg 2 domain Carbohydrate is essential for complement activation Subtly different hinge regions between subclasses accounts for differing abilities to activate complement Ig. G 3>Ig. G 1>Ig. G 2
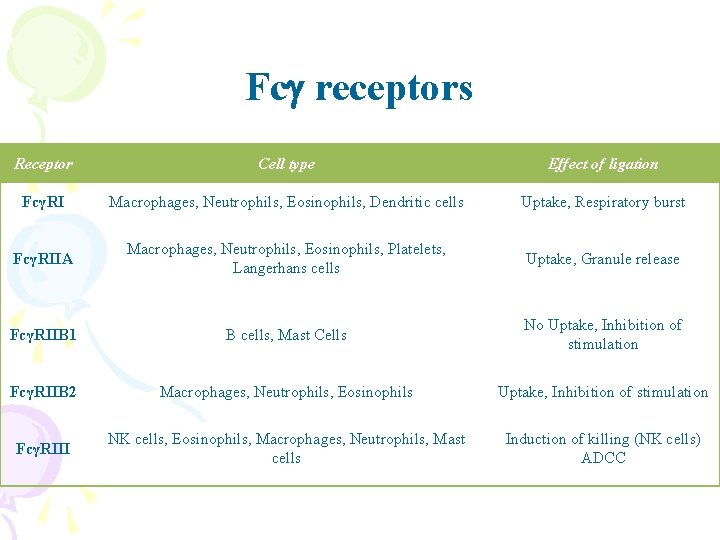
Fcg receptors Receptor Cell type Effect of ligation FcγRI Macrophages, Neutrophils, Eosinophils, Dendritic cells Uptake, Respiratory burst FcγRIIA Macrophages, Neutrophils, Eosinophils, Platelets, Langerhans cells Uptake, Granule release FcγRIIB 1 B cells, Mast Cells No Uptake, Inhibition of stimulation FcγRIIB 2 Macrophages, Neutrophils, Eosinophils Uptake, Inhibition of stimulation FcγRIII NK cells, Eosinophils, Macrophages, Neutrophils, Mast cells Induction of killing (NK cells) ADCC
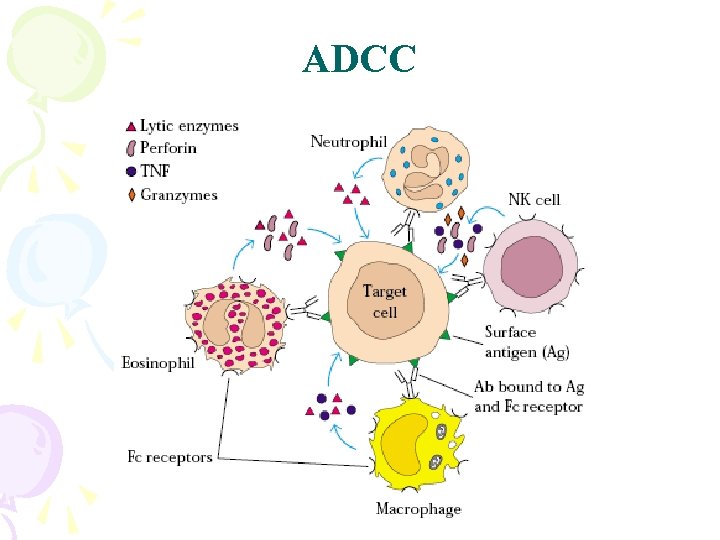
ADCC
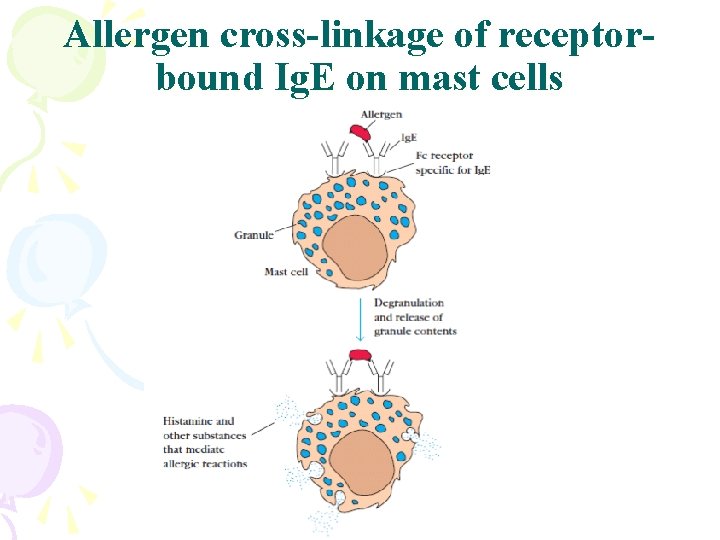
Allergen cross-linkage of receptorbound Ig. E on mast cells
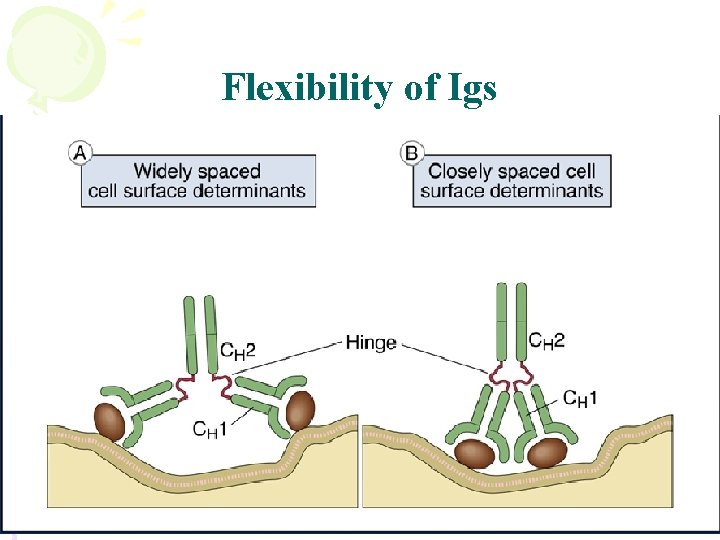
Flexibility of Igs
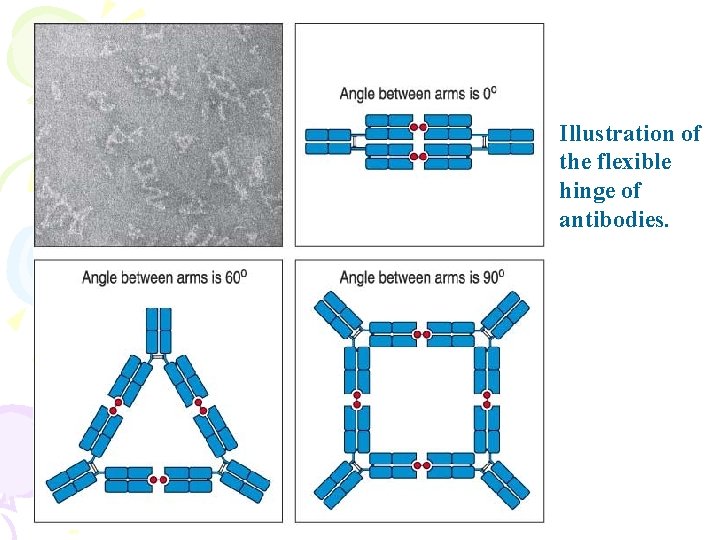
Illustration of the flexible hinge of antibodies.
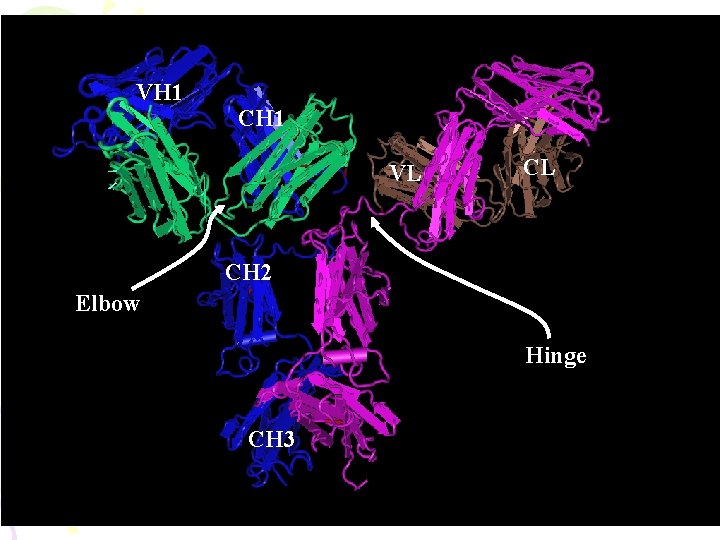
VH 1 CH 1 VL CL CH 2 Elbow Hinge CH 3
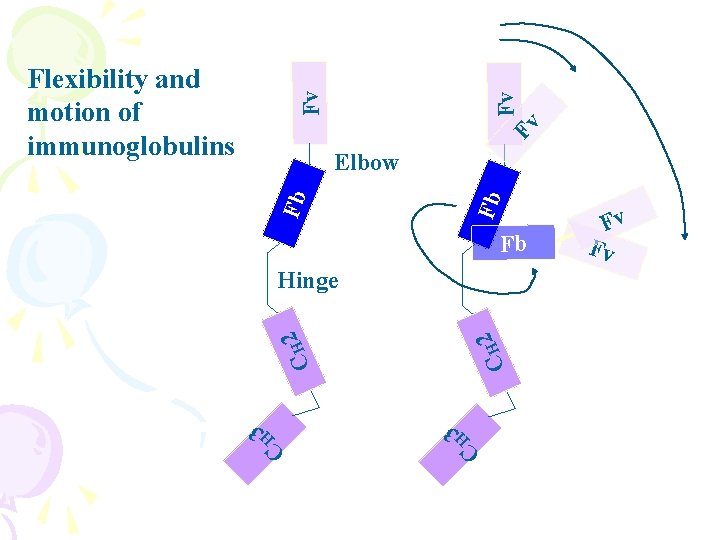
Fv Fv Fv Flexibility and motion of immunoglobulins Fb Fb Elbow Fb 2 CH 3 H C C H 3 2 CH Hinge Fv Fv
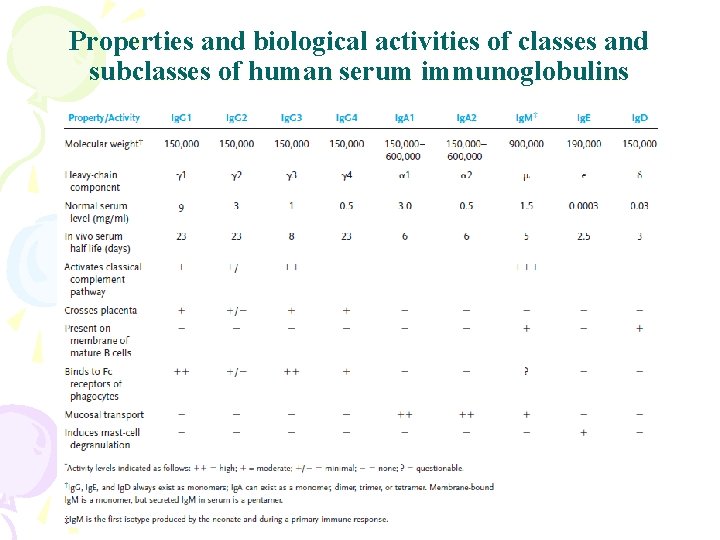
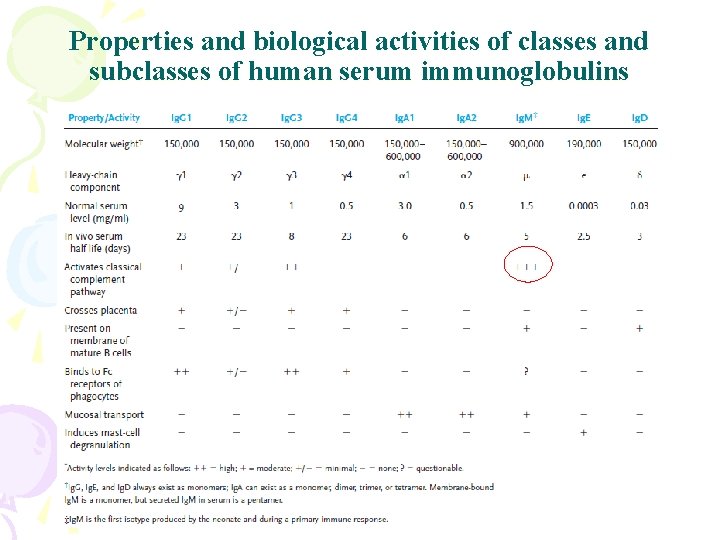
Properties and biological activities of classes and subclasses of human serum immunoglobulins
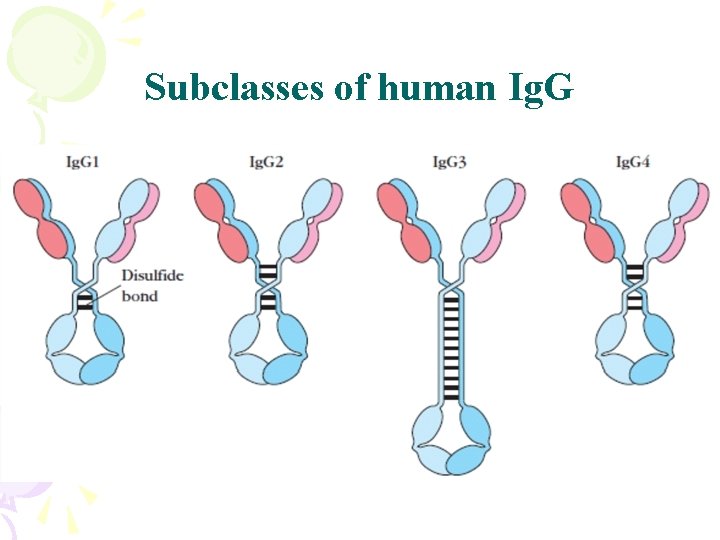
Subclasses of human Ig. G

Properties of Ig. G • Major anti-infection Ig – Major Ig in serum and extravascular spaces – Have the longest half life – Fixes complement ( Ig. G 4) – Binds to Fc receptors • Phagocytes - opsonization • NK cells – ADCC • Placental transfer – Does not require Ag binding
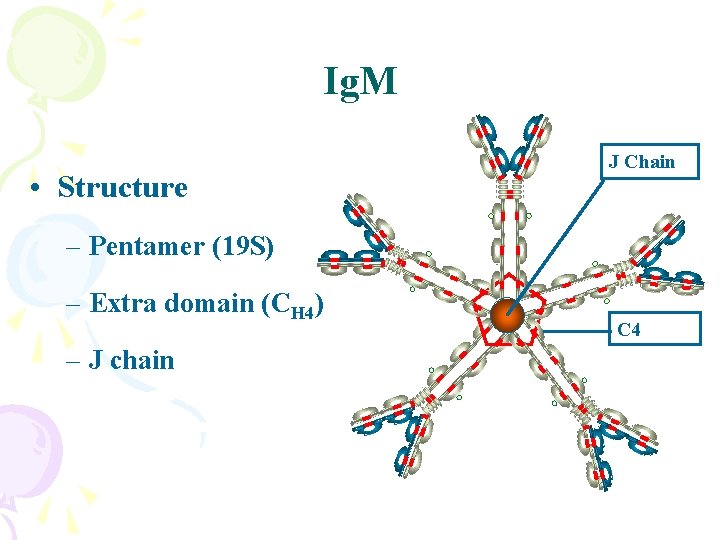
Ig. M • Structure J Chain – Pentamer (19 S) – Extra domain (CH 4) – J chain C 4
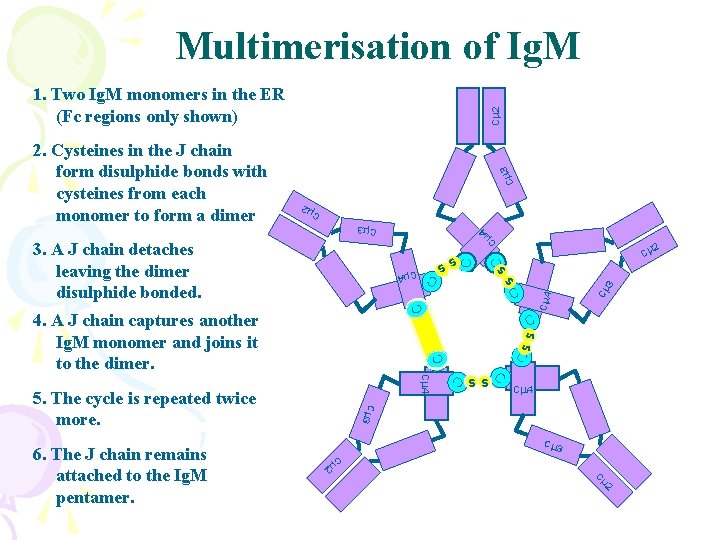
Multimerisation of Ig. M 3 Cm Cm 2 C Cm 4 C Cm 3 s C C C CC ss C CC C C Cm 4 ss Cm 4 Cm 3 5. The cycle is repeated twice more. 2 Cm s. C Cm 4 4. A J chain captures another Ig. M monomer and joins it to the dimer. Cm 3 C m 2 6. The J chain remains attached to the Ig. M pentamer. C ss 3. A J chain detaches leaving the dimer disulphide bonded. m 4 Cm 3 2. Cysteines in the J chain form disulphide bonds with cysteines from each monomer to form a dimer Cm 2 1. Two Ig. M monomers in the ER (Fc regions only shown)

Ig. M • Properties – First Ig made by fetus and B cells – The most effective complement activator – Agglutinate Ig – B cell surface Ig – Natural occuring Abs against ABO blood group antigens
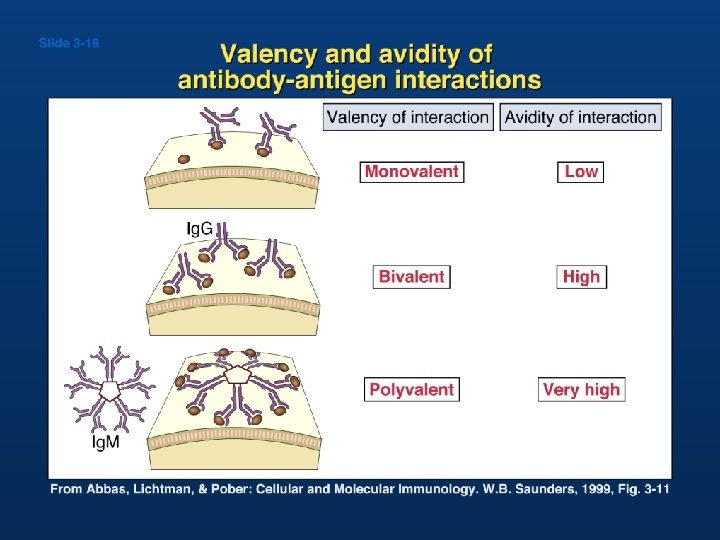
Valency
Properties and biological activities of classes and subclasses of human serum immunoglobulins
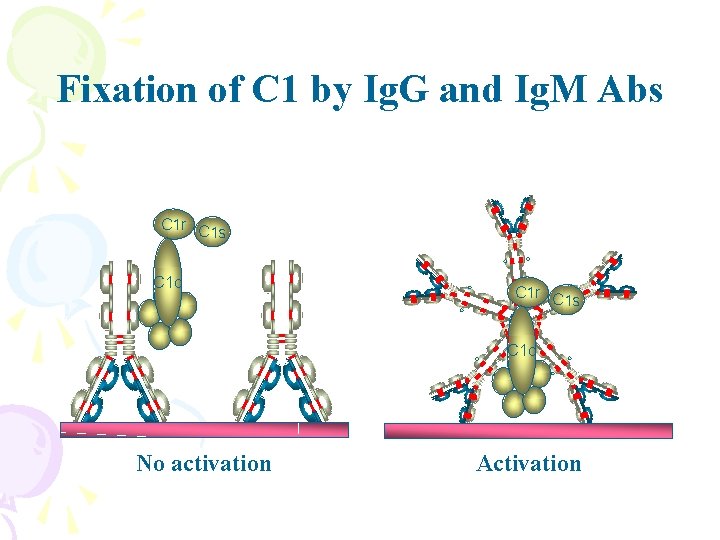
Fixation of C 1 by Ig. G and Ig. M Abs C 1 r C 1 s C 1 q No activation Activation
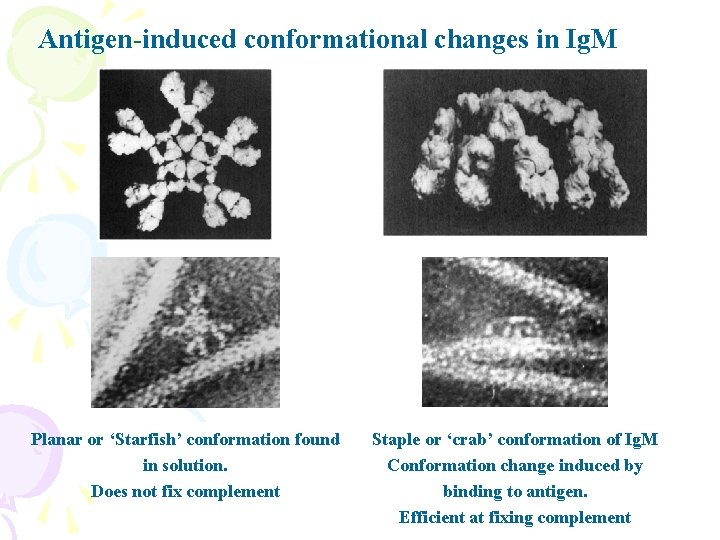
Antigen-induced conformational changes in Ig. M Planar or ‘Starfish’ conformation found in solution. Does not fix complement Staple or ‘crab’ conformation of Ig. M Conformation change induced by binding to antigen. Efficient at fixing complement
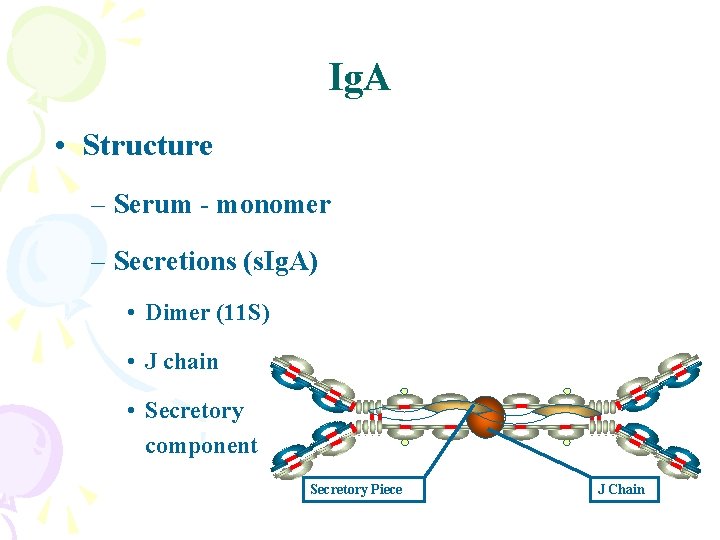
Ig. A • Structure – Serum - monomer – Secretions (s. Ig. A) • Dimer (11 S) • J chain • Secretory component Secretory Piece J Chain

Ig. A • Properties – Major secretory Ig (Mucosal or Local Immunity) • Tears, saliva, gastric and pulmonary secretions – Does not fix complement (unless aggregated) – Binds to Fc receptors on some cells
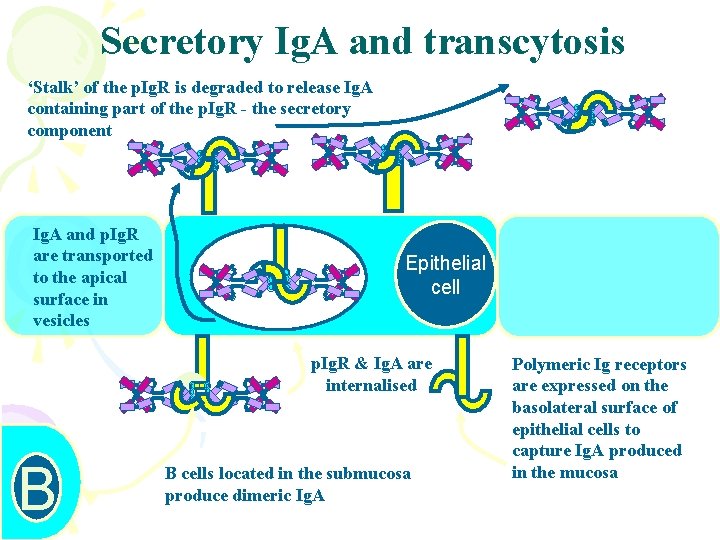
Secretory Ig. A and transcytosis S S SS C J C C ss C S S SS S S B C J C C ss C S S SS CJC C C Css. C S S SS C J C C ss C Ig. A and p. Ig. R are transported to the apical surface in vesicles SS ‘Stalk’ of the p. Ig. R is degraded to release Ig. A containing part of the p. Ig. R - the secretory component Epithelial cell p. Ig. R & Ig. A are internalised SS B cells located in the submucosa produce dimeric Ig. A Polymeric Ig receptors are expressed on the basolateral surface of epithelial cells to capture Ig. A produced in the mucosa

Immune benefits of breast milk breast-feeding
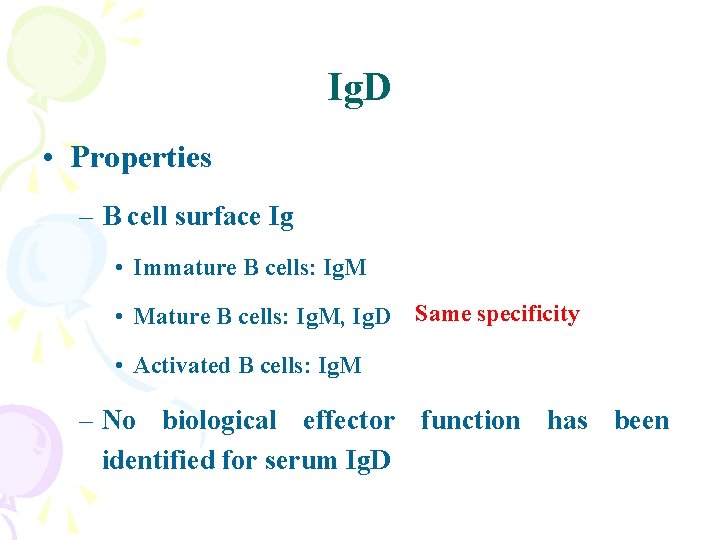
Ig. D • Properties – B cell surface Ig • Immature B cells: Ig. M • Mature B cells: Ig. M, Ig. D Same specificity • Activated B cells: Ig. M – No biological effector function has been identified for serum Ig. D
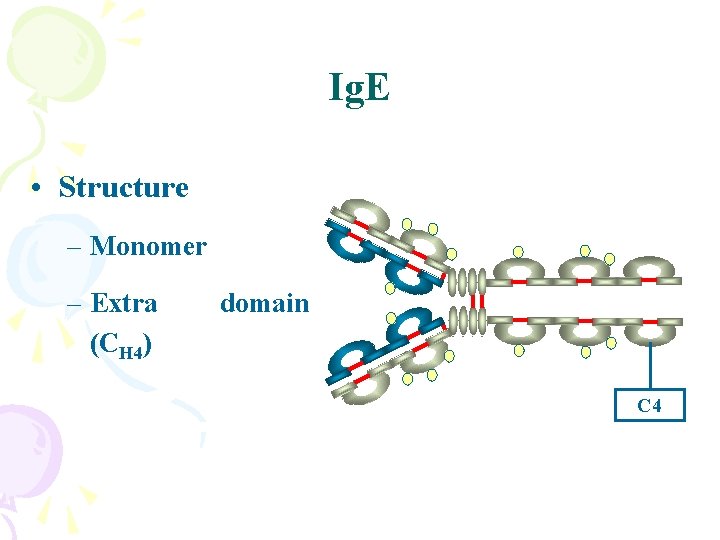
Ig. E • Structure – Monomer – Extra (CH 4) domain C 4
Ig. E • Properties – Least common serum Ig • Binds to basophils and mast cells (Does not require Ag binding) – Allergic reactions – Parasitic infections (Helminths) • Binds to Fc receptor on eosinophils
Monoclonal antibody • Monoclonal antibody: derived from a single B-cell clone and thus specific for a single epitope; • Polyclonal antibody: derived from multiple B-cell clones and the resulting serum antibodies are heterogeneous, comprising a mixture of monoclonal antibodies.
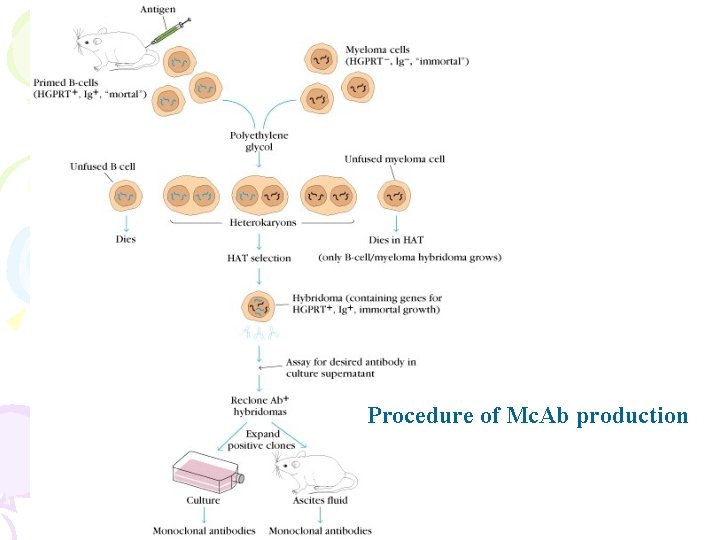
Procedure of Mc. Ab production

Remaining problems • How can our body produce huge amount of Abs? • Can Mc. Abs be uesd in human directly? —— Antigenic determinants on Igs
- Slides: 56