IMI an opportunity to implement research for patients












- Slides: 14

IMI – an opportunity to implement research for patients and with patients Catherine Brett European Parliament Interest Group on Allergy and Asthma 09. 10. 2018 • Brussels, Belgium

Outline § What is IMI? § How are patients involved? § U-BIOPRED § EUPATI § PARADIGM § IMI and patients – an evolving story § An invitation!

What is IMI?

IMI – Europe’s partnership for health > € 5 bn € 2. 5 bn Partnership 2008 - 2020 € 2. 5 bn

Goals of IMI 2 programme ü Improve the drug development process by creating tools to assess the efficacy, safety and quality of medicines ü Develop biological markers to diagnose diseases and assess treatments ü Speed up the earlier stages of drug development ü Increase the success rate of clinical trials of priority medicines ü Develop new therapies for diseases with a high unmet need / limited market incentives ü Reduce the failure rate of vaccine candidates in clinical trials

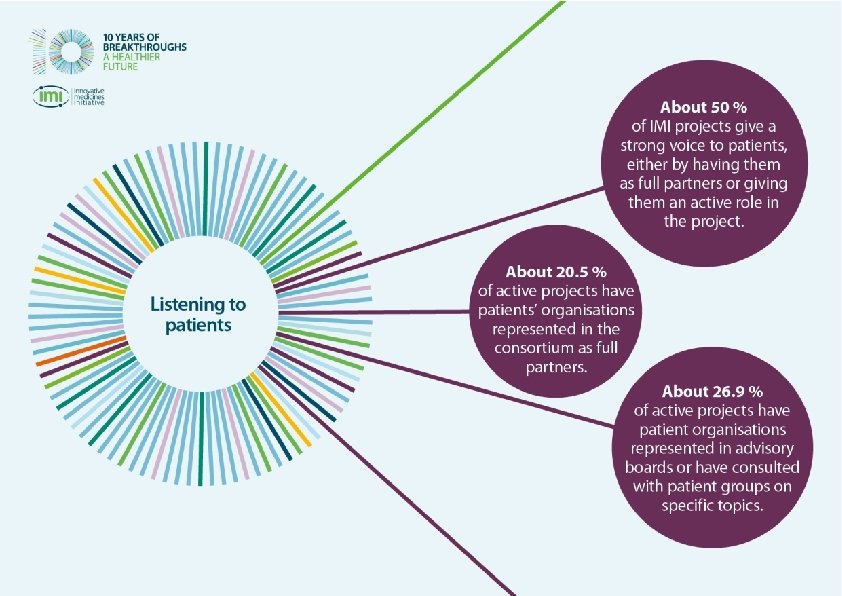
How are patients involved in IMI?


U-BIOPRED – breathing new life into severe asthma research The biggest and most complex research project undertaken to understand severe asthma to date. Main achievements: § uncovered a number of subtypes of severe asthma – this is already paving the way for the development of more effective treatments; § registry with data from 1 025 patients including children & adults; § biobank with 50 000+ severe asthma patient samples (e. g. blood, sputum, lung tissue, urine), available for future research; § exemplary patient involvement! (and lessons being shared).

EUPATI graduates - making a difference § 90+ graduates § 58 disease areas, 31 countries § More & deeper involvement in R&D thanks to EUPATI ü Sustainability (course, toolbox maintained by EPF) ‘Now we can speak with conviction & determination’ - Lara Stone

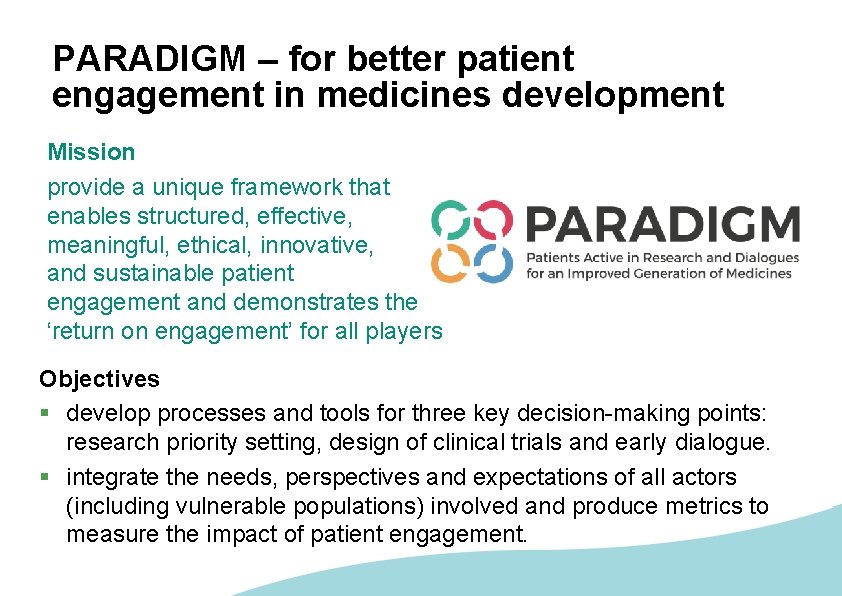
PARADIGM – for better patient engagement in medicines development Mission provide a unique framework that enables structured, effective, meaningful, ethical, innovative, and sustainable patient engagement and demonstrates the ‘return on engagement’ for all players Objectives § develop processes and tools for three key decision-making points: research priority setting, design of clinical trials and early dialogue. § integrate the needs, perspectives and expectations of all actors (including vulnerable populations) involved and produce metrics to measure the impact of patient engagement.

IMI and patients

IMI and patients - an evolving story ü ü ü ü Patient-focused projects Patients as full IMI project partners (receiving funding) Patients on project committees Patients as IMI 2 Associated Partners (contributing resources) Patients on IMI Scientific Committee Patients as speakers at IMI events Hired a full-time patient engagement expert - NEW Current goals: Ø Design & implement framework for deeper, more meaningful patient engagement in IMI.

Join us for our Scientific Symposium & Stakeholder Forum IMI Scientific Symposium Towards personalised medicines | Patient-centric approaches | Enablers for drug discovery & development | Collaborating to fight infections 22 -23 October 2018 IMI Stakeholder Forum The value of cross-sector health research & innovation 24 October 2018 Brussels, Belgium Registration via www. imi. europa. eu #IMITen. Years #IMICarry. The. Torc h

Thank you Catherine Brett • External Relations Manager catherine. brett@imi. europa. eu www. imi. europa. eu @IMI_JU