IMF Practice You will first need to determine


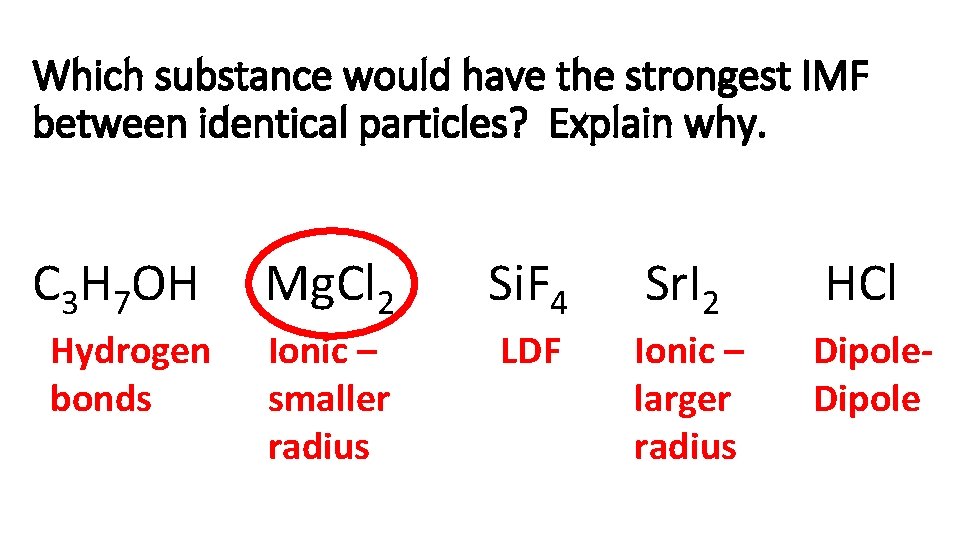


- Slides: 13

IMF Practice You will first need to determine if the substance is ionic or covalent. If covalent, plan on drawing the molecule to determine molecular polarity!!!

Which type of intermolecular forces is caused by an instantaneous dipole between particles? London Dispersion Forces

Identify the IMF present in a sample of each of the following substances. Copper and Zinc mixture Metallic London Dispersion Forces CS 2 CH 3 NH 2 Hydrogen bonding Dipole-dipole PH 3 Pb. O 2 Ionic

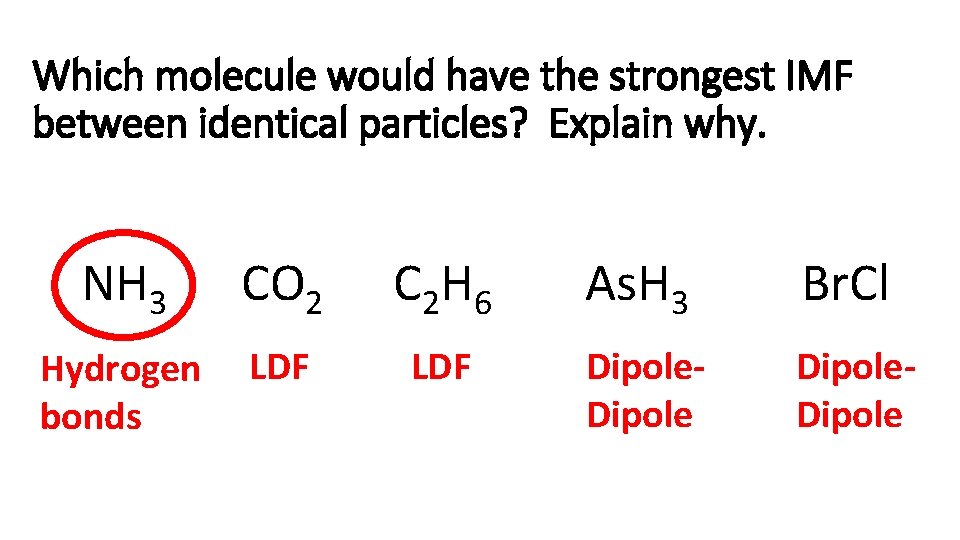
Which molecule would have the strongest IMF between identical particles? Explain why. NH 3 CO 2 C 2 H 6 As. H 3 Br. Cl Hydrogen bonds LDF Dipole
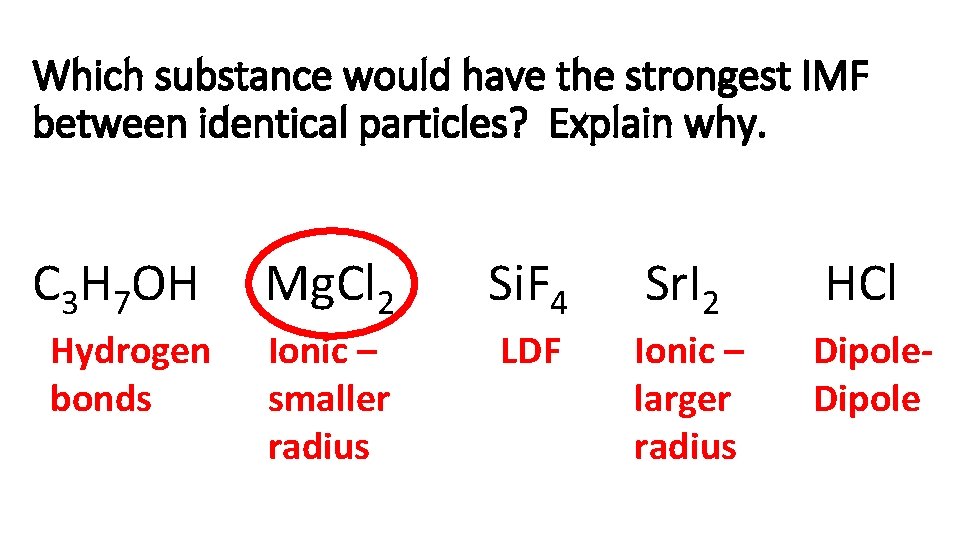
Which substance would have the strongest IMF between identical particles? Explain why. C 3 H 7 OH Mg. Cl 2 Si. F 4 Sr. I 2 HCl Hydrogen bonds Ionic – smaller radius LDF Ionic – larger radius Dipole

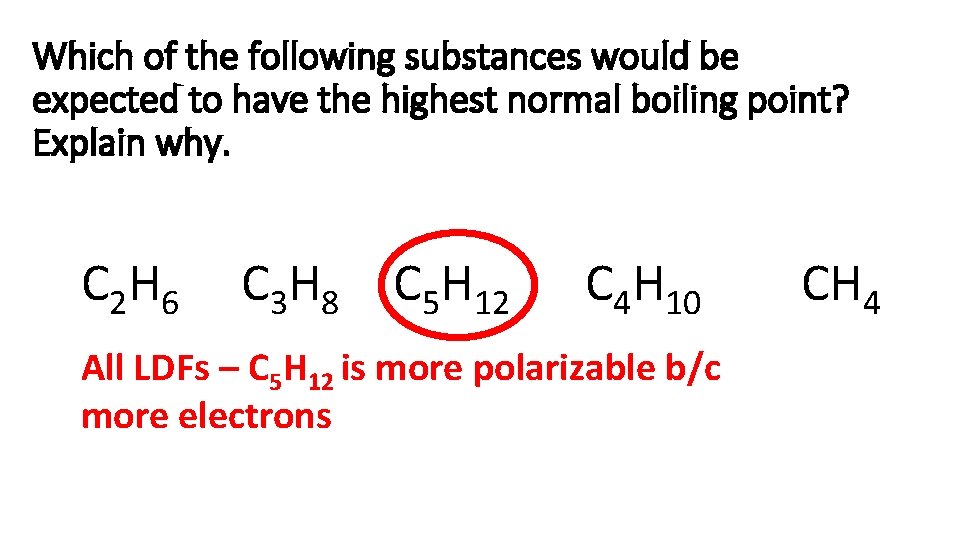
Which of the following substances would be expected to have the highest normal boiling point? Explain why. C 2 H 6 C 3 H 8 C 5 H 12 C 4 H 10 All LDFs – C 5 H 12 is more polarizable b/c more electrons CH 4

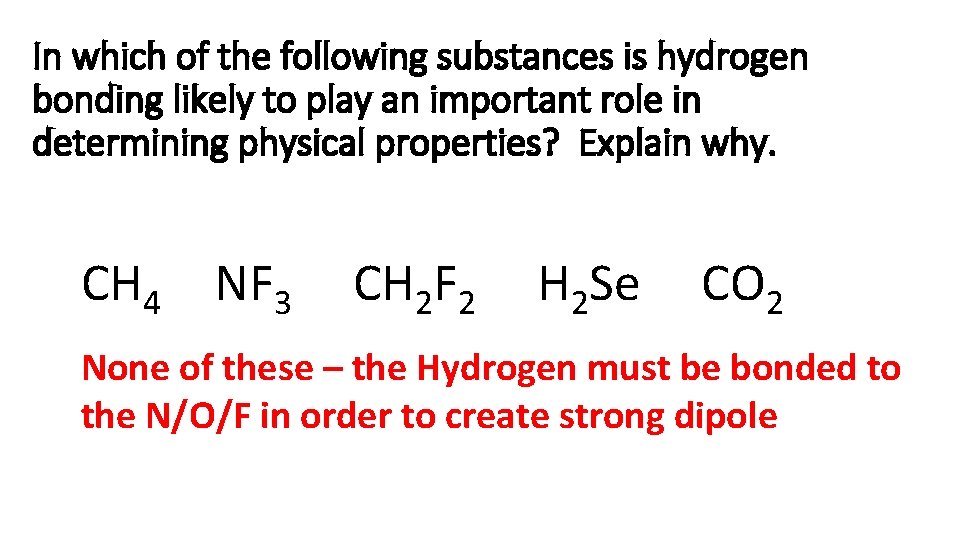
In which of the following substances is hydrogen bonding likely to play an important role in determining physical properties? Explain why. CH 4 NF 3 CH 2 F 2 H 2 Se CO 2 None of these – the Hydrogen must be bonded to the N/O/F in order to create strong dipole

List the substances Ba. Cl 2, O 2, Ag, HF, and PF 3 in order of increasing boiling points. Explain your answer. O 2 (lowest; LDF only) PF 3 (dipole – dipole) HF (hydrogen bond) Ag (metallic) Ba. Cl 2 (highest; ionic)

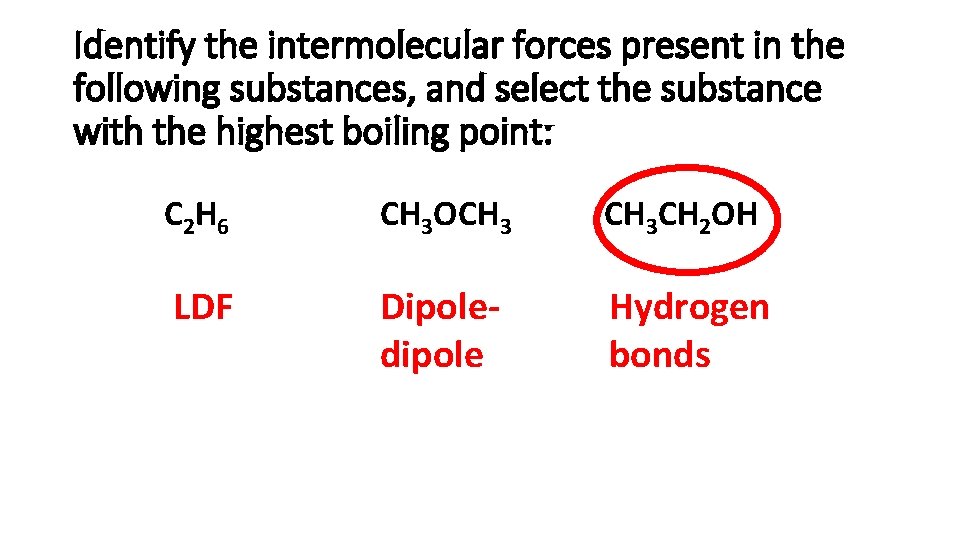
Identify the intermolecular forces present in the following substances, and select the substance with the highest boiling point: C 2 H 6 CH 3 OCH 3 CH 2 OH LDF Dipoledipole Hydrogen bonds

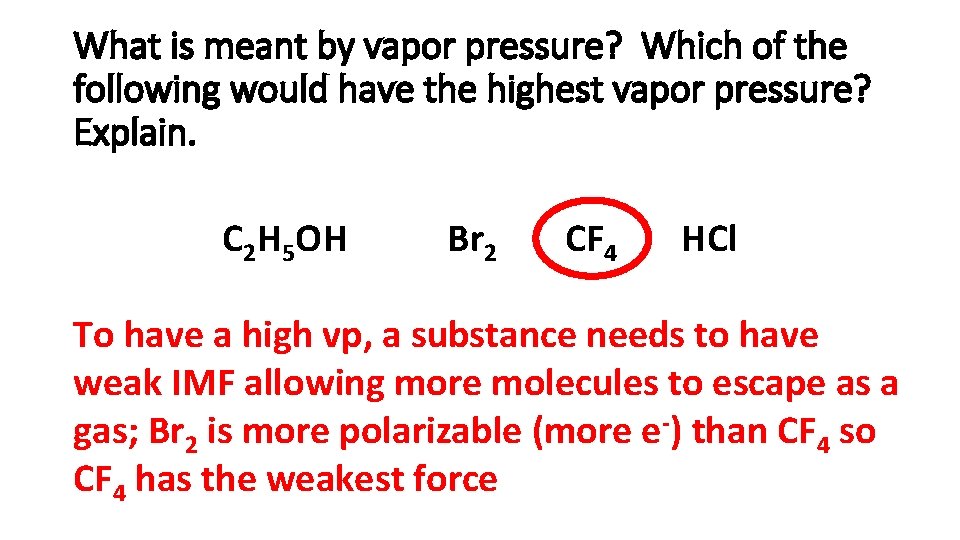
What is meant by vapor pressure? Which of the following would have the highest vapor pressure? Explain. C 2 H 5 OH Br 2 CF 4 HCl To have a high vp, a substance needs to have weak IMF allowing more molecules to escape as a gas; Br 2 is more polarizable (more e-) than CF 4 so CF 4 has the weakest force

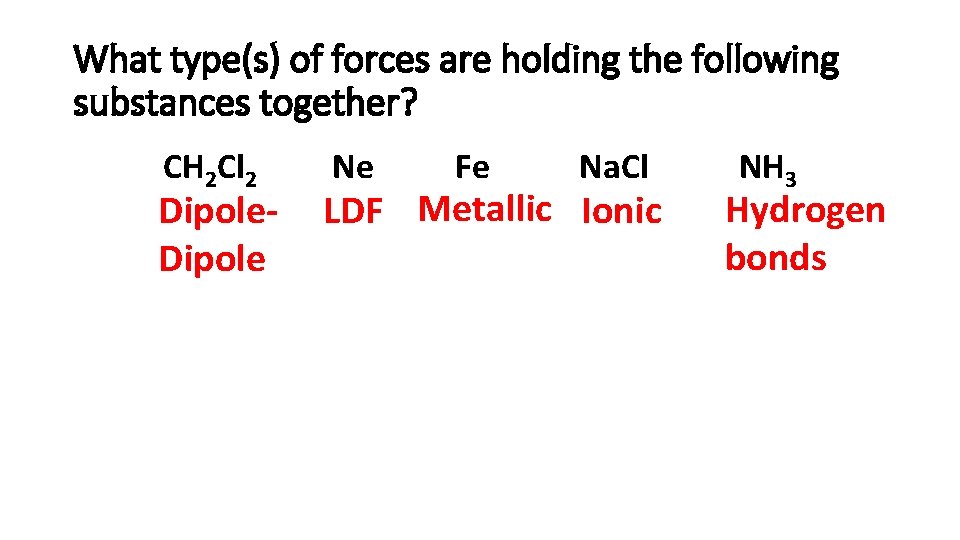
What type(s) of forces are holding the following substances together? CH 2 Cl 2 Dipole Ne Fe Na. Cl LDF Metallic Ionic NH 3 Hydrogen bonds

Draw a particulate diagram of liquid water. Your drawing must include multiple water molecules. Label a covalent bond, an intramolecular bond an intermolecular bond.

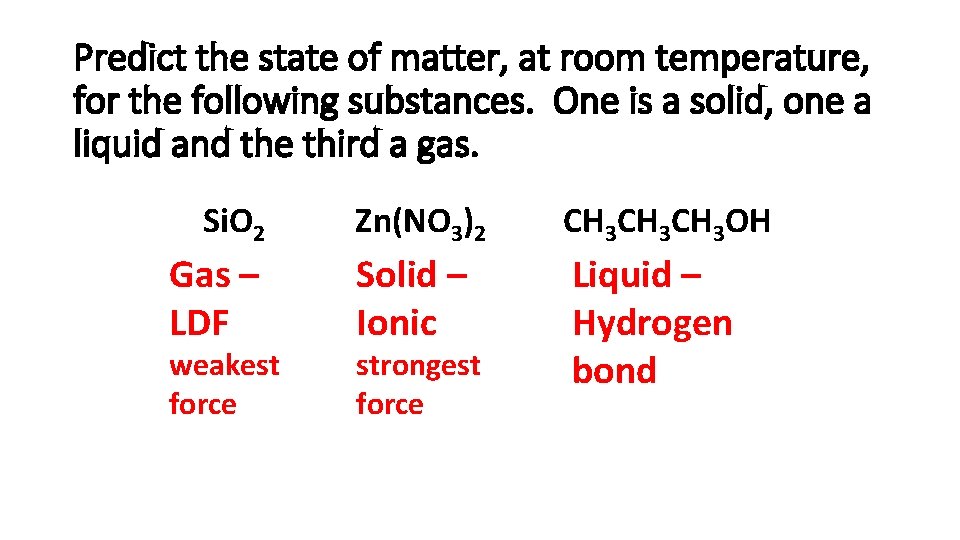
Predict the state of matter, at room temperature, for the following substances. One is a solid, one a liquid and the third a gas. Si. O 2 Gas – LDF weakest force Zn(NO 3)2 CH 3 CH 3 OH Solid – Ionic Liquid – Hydrogen bond strongest force