Imaging in Dementia Options for Clinical Practice 2016









![Amyloid Imaging: Pittsburgh Compound-B PET T 1 W-MRI Radiolabeled thioflavin derivative n [N-methyl-(11)C]2 -(4’methylaminophenyl)-6 Amyloid Imaging: Pittsburgh Compound-B PET T 1 W-MRI Radiolabeled thioflavin derivative n [N-methyl-(11)C]2 -(4’methylaminophenyl)-6](https://slidetodoc.com/presentation_image/309139a07eedd31384e1d5b6a9816a79/image-30.jpg)













- Slides: 68

Imaging in Dementia: Options for Clinical Practice 2016 John A. Bertelson, MD Clinical Chief of Neurology, Seton Brain and Spine Institute Assistant Professor of Medicine, Dell Medical School, UT Austin Clinical Assistant Professor of Psychology, UT Austin

Disclosures n None

Outline n Early Imaging n Indications for Imaging in Dementia n Imaging of Alzheimer’s Disease n Imaging of Other Dementing Disorders n Future Directions

Early Imaging Modality What am I? n Initially described in 1918 1 n n Low resolution High morbidity, including: n Meningeal irritation, 6 hrs: n n n Headache Nausea Emesis Elevation in BP Became obsolete in 1971 1: AJNR 2012

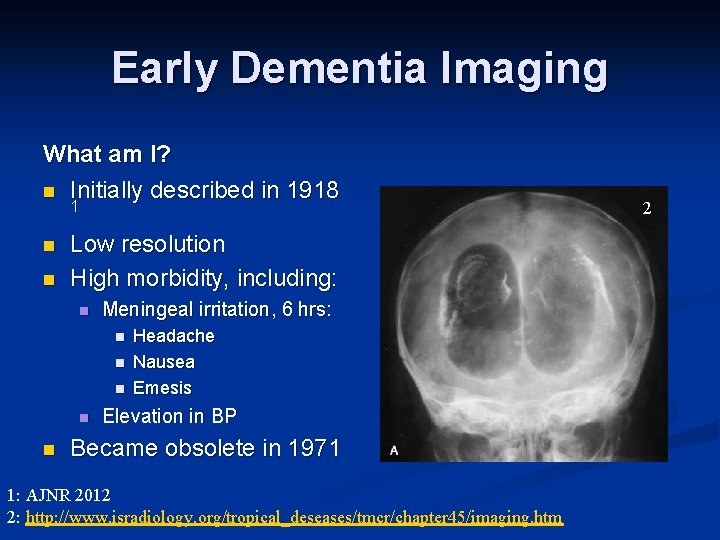
Early Dementia Imaging What am I? n Initially described in 1918 1 n n Low resolution High morbidity, including: n Meningeal irritation, 6 hrs: n n n Headache Nausea Emesis Elevation in BP Became obsolete in 1971 1: AJNR 2012 2: http: //www. isradiology. org/tropical_deseases/tmcr/chapter 45/imaging. htm 2

Early Dementia Imaging What am I? n Initially described in 1918 Pneumoencephalogra phy 1 n n Low resolution High morbidity, including: n Meningeal irritation, 6 hrs: n n n Headache Nausea Emesis Elevation in BP Became obsolete in 1971 1: AJNR 2012 2: http: //www. isradiology. org/tropical_deseases/tmcr/chapter 45/imaging. htm 2

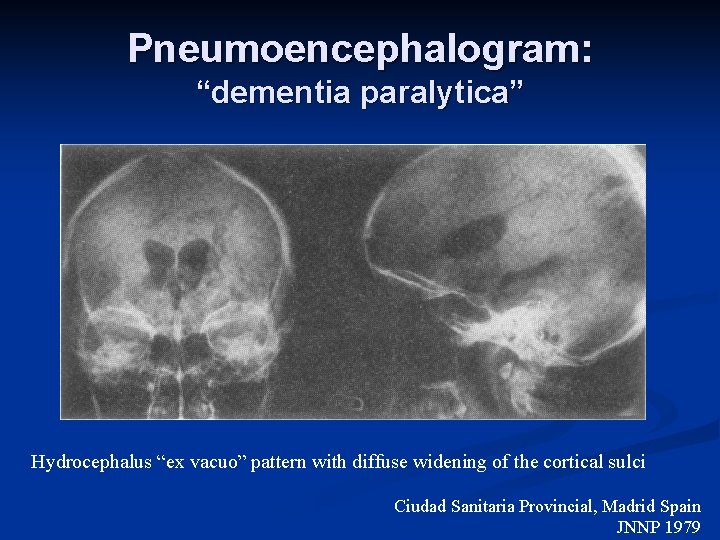
Pneumoencephalogram: “dementia paralytica” Hydrocephalus “ex vacuo” pattern with diffuse widening of the cortical sulci Ciudad Sanitaria Provincial, Madrid Spain JNNP 1979

Modern Imaging

Traditional Role for Neuroimaging in Dementia n Indication: Rule out reversible process n Quality Standards Subcommittee of the AAN, 1994: n “Neuroimaging should be considered in every patient with dementia” n “… potentially treatable disorders that can otherwise be missed, such as tumors, subdural hematomas, hydrocephalus, and strokes. ” n “… there is no consensus on the need for such studies in the evaluation of patients with the insidious onset of dementia after age 60 without focal signs or symptoms, seizures, or gait disturbances. ” Alter M, 1994

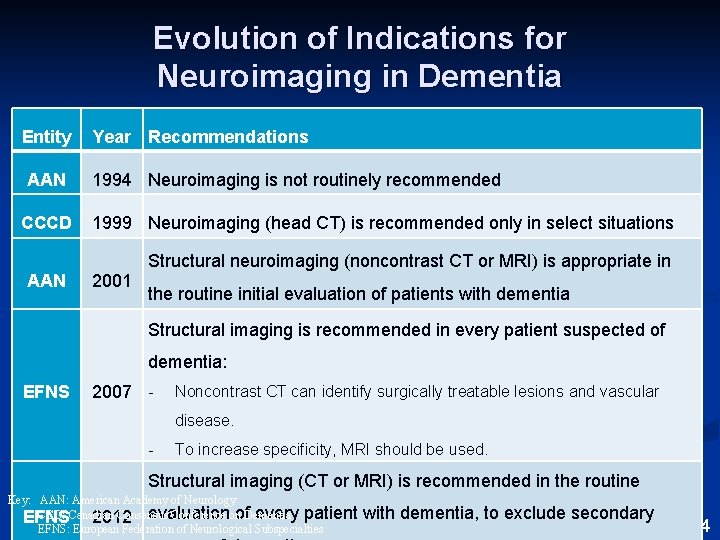
Evolution of Indications for Neuroimaging in Dementia Entity AAN CCCD AAN Year Recommendations 1994 Neuroimaging is not routinely recommended 1999 Neuroimaging (head CT) is recommended only in select situations 2001 Structural neuroimaging (noncontrast CT or MRI) is appropriate in the routine initial evaluation of patients with dementia Structural imaging is recommended in every patient suspected of dementia: EFNS 2007 - Noncontrast CT can identify surgically treatable lesions and vascular disease. - To increase specificity, MRI should be used. Structural imaging (CT or MRI) is recommended in the routine Key: AAN: American Academy of Neurology CCD: Canadian Consensus Conference on evaluation of. Dementia every patient EFNS 2012 EFNS: European Federation of Neurological Subspecialties with dementia, to exclude secondary From: Bertelson and Ajtai, 2014

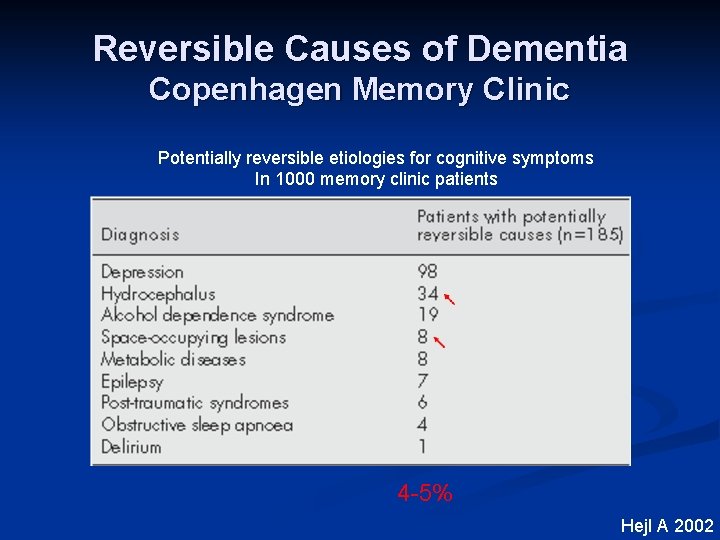
Reversible Causes of Dementia Copenhagen Memory Clinic Potentially reversible etiologies for cognitive symptoms In 1000 memory clinic patients 4 -5% Hejl A 2002

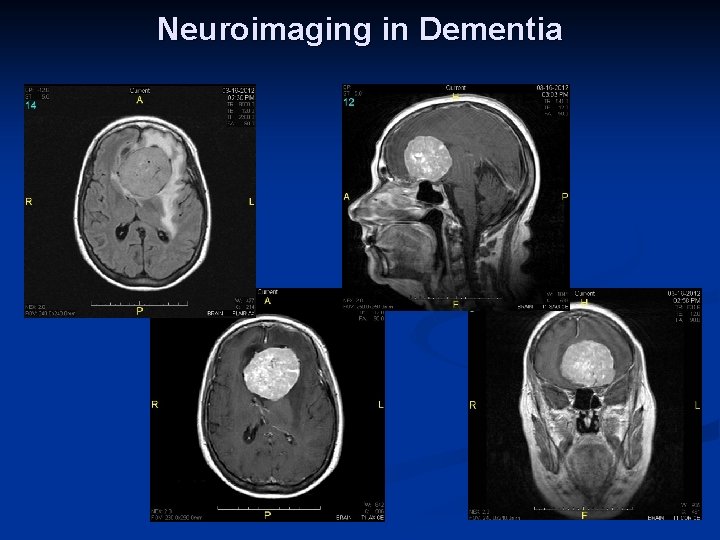
Neuroimaging in Dementia

Alzheimer’s Disease (AD)

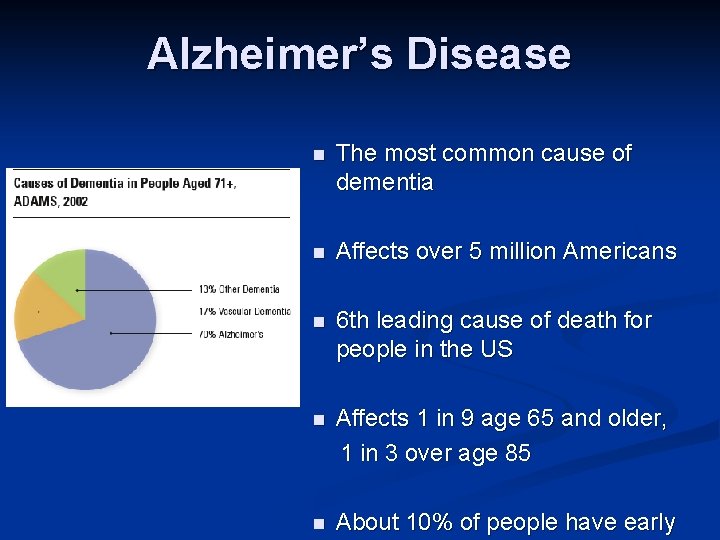
Alzheimer’s Disease n The most common cause of dementia n Affects over 5 million Americans n 6 th leading cause of death for people in the US n Affects 1 in 9 age 65 and older, 1 in 3 over age 85 n About 10% of people have early

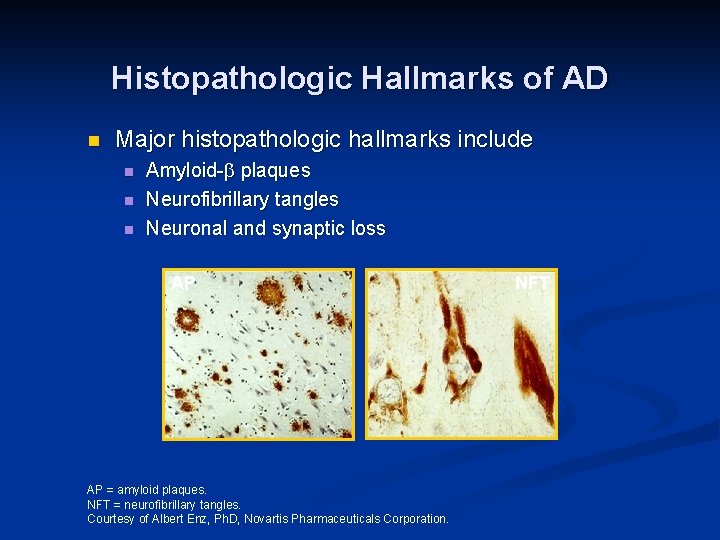
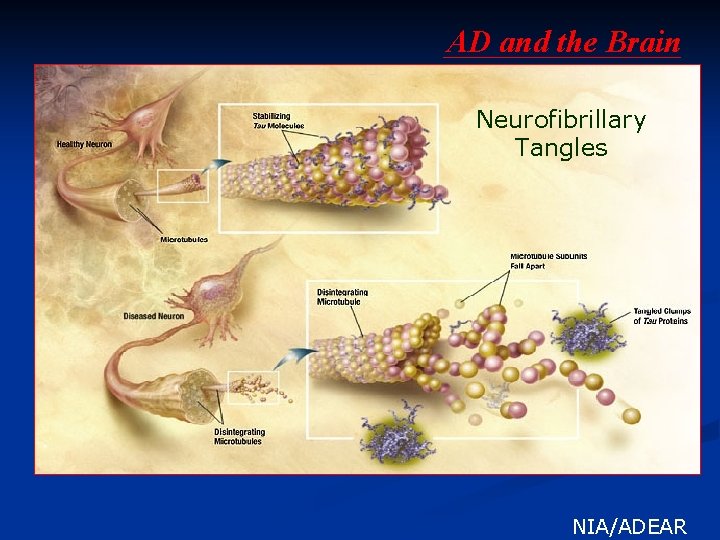
Histopathologic Hallmarks of AD n Major histopathologic hallmarks include n n n Amyloid- plaques Neurofibrillary tangles Neuronal and synaptic loss AP AP = amyloid plaques. NFT = neurofibrillary tangles. Courtesy of Albert Enz, Ph. D, Novartis Pharmaceuticals Corporation. NFT

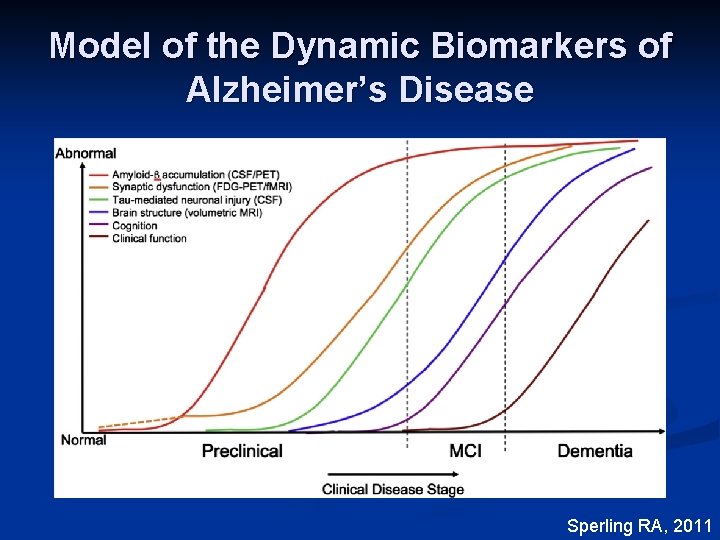
Model of the Dynamic Biomarkers of Alzheimer’s Disease Sperling RA, 2011

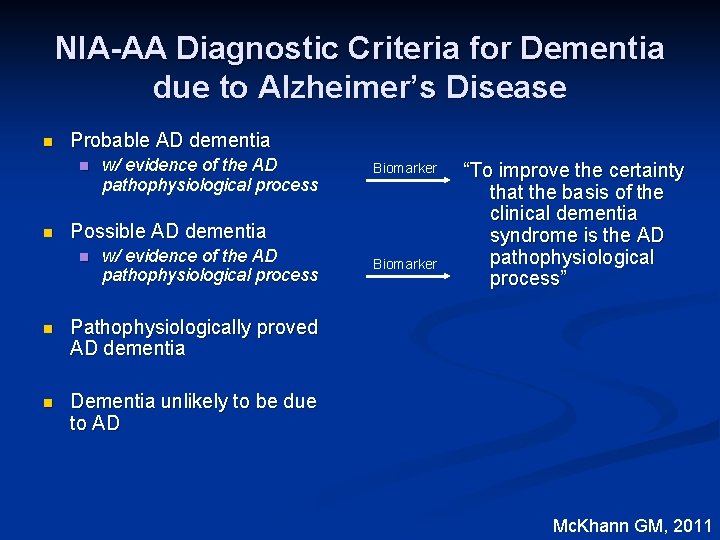
NIA-AA Diagnostic Criteria for Dementia due to Alzheimer’s Disease n Probable AD dementia n Possible AD dementia n Pathophysiologically proved AD dementia n Dementia unlikely to be due to AD Mc. Khann GM, 2011

NIA-AA Diagnostic Criteria for Dementia due to Alzheimer’s Disease n Probable AD dementia n n w/ evidence of the AD pathophysiological process Biomarker Possible AD dementia n w/ evidence of the AD pathophysiological process n Pathophysiologically proved AD dementia n Dementia unlikely to be due to AD Biomarker “To improve the certainty that the basis of the clinical dementia syndrome is the AD pathophysiological process” Mc. Khann GM, 2011

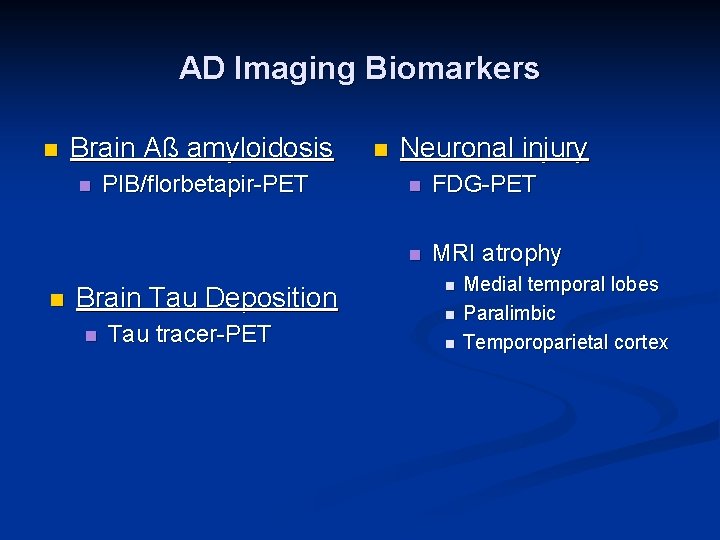
AD Imaging Biomarkers n Brain Aß amyloidosis n n PIB/florbetapir-PET Brain Tau Deposition n Tau tracer-PET n Neuronal injury n FDG-PET n MRI atrophy n n n Medial temporal lobes Paralimbic Temporoparietal cortex

MRI and AD

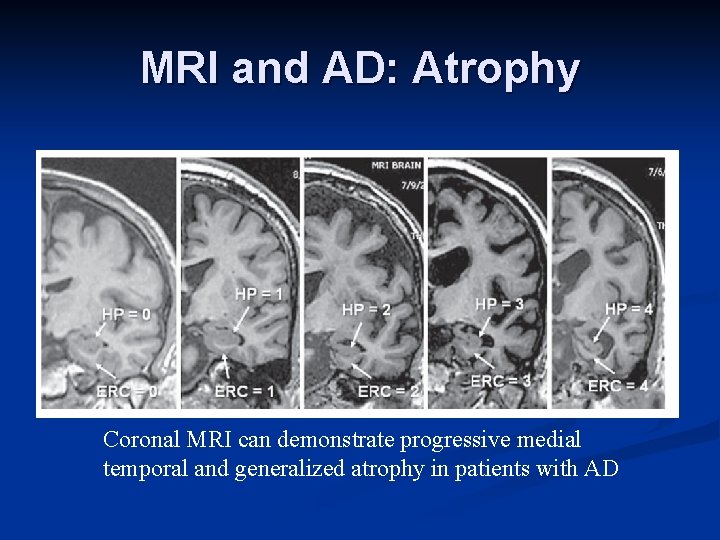
MRI and AD: Atrophy Coronal MRI can demonstrate progressive medial temporal and generalized atrophy in patients with AD

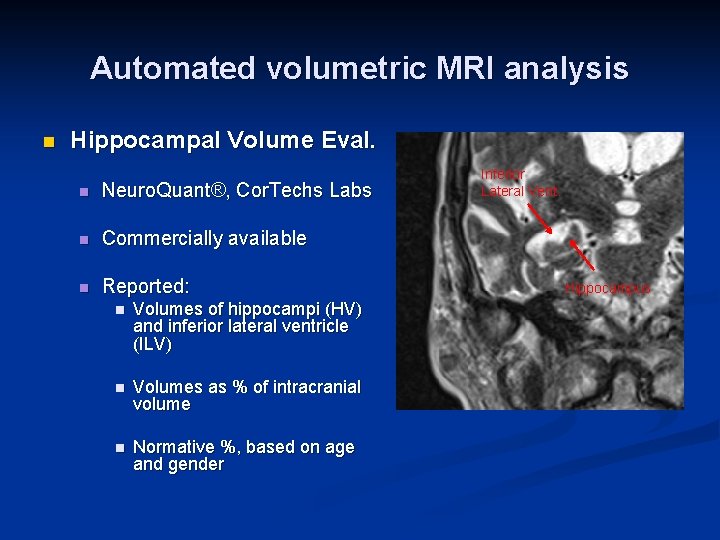
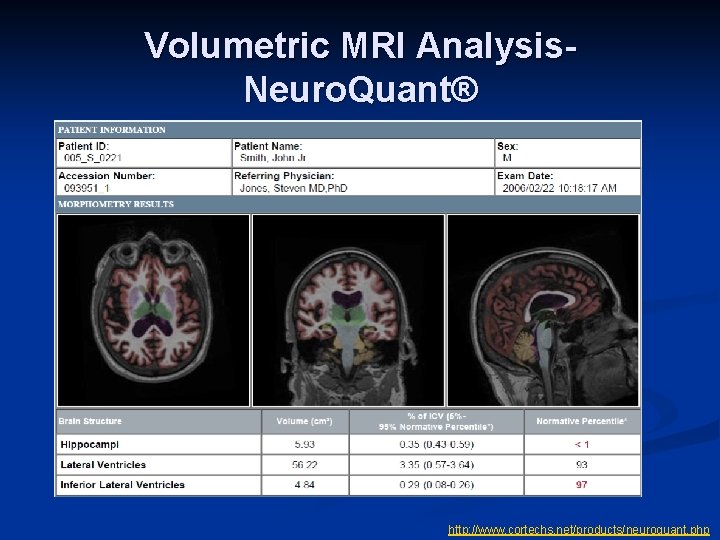
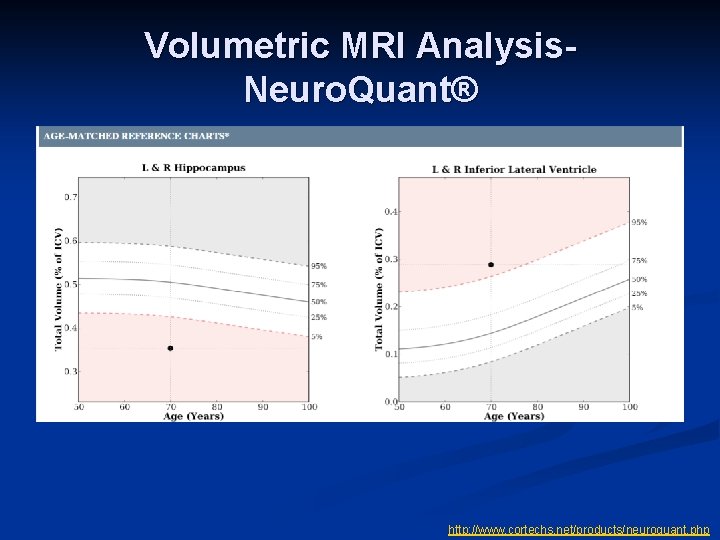
Automated volumetric MRI analysis n Hippocampal Volume Eval. n Neuro. Quant®, Cor. Techs Labs n Commercially available n Reported: n Volumes of hippocampi (HV) and inferior lateral ventricle (ILV) n Volumes as % of intracranial volume n Normative %, based on age and gender Inferior Lateral Vent. Hippocampus

Volumetric MRI Analysis. Neuro. Quant® http: //www. cortechs. net/products/neuroquant. php

Volumetric MRI Analysis. Neuro. Quant® http: //www. cortechs. net/products/neuroquant. php

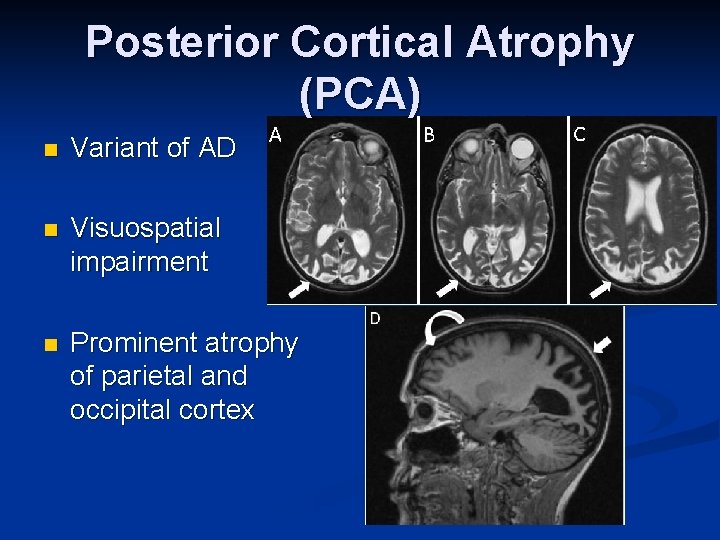
Posterior Cortical Atrophy (PCA) n Variant of AD n Visuospatial impairment n Prominent atrophy of parietal and occipital cortex

PET and AD n FDG PET n Amyloid PET n Tau PET

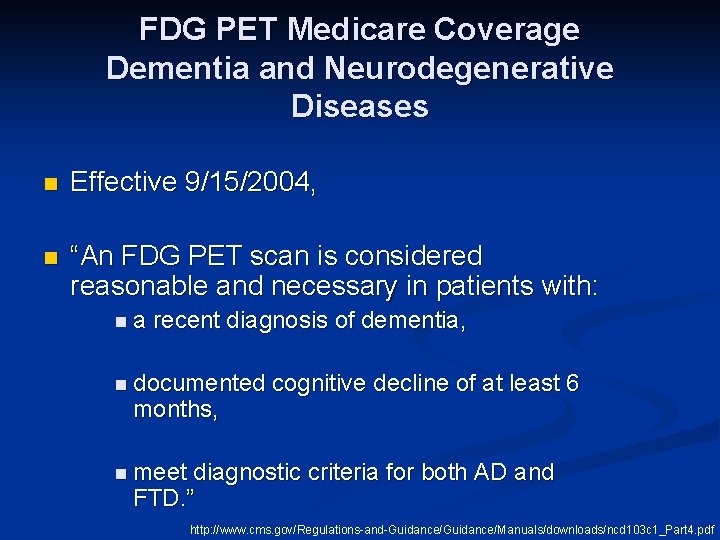
FDG PET Medicare Coverage Dementia and Neurodegenerative Diseases n Effective 9/15/2004, n “An FDG PET scan is considered reasonable and necessary in patients with: na recent diagnosis of dementia, n documented months, cognitive decline of at least 6 n meet diagnostic criteria for both AD and FTD. ” http: //www. cms. gov/Regulations-and-Guidance/Manuals/downloads/ncd 103 c 1_Part 4. pdf

FDG PET Medicare Coverage Dementia and Neurodegenerative Diseases n Additional prerequisites include: n Comprehensive evaluation already completed, including brain CT or MRI n Evaluation by “a physician experienced in the diagnosis and assessment of dementia” n Evaluation is indeterminate and FDG PET is reasonably expected to clarify the diagnosis between FTD and AD n SPECT or PET have not already been obtained in the past 12 months AND significant clinical changes have

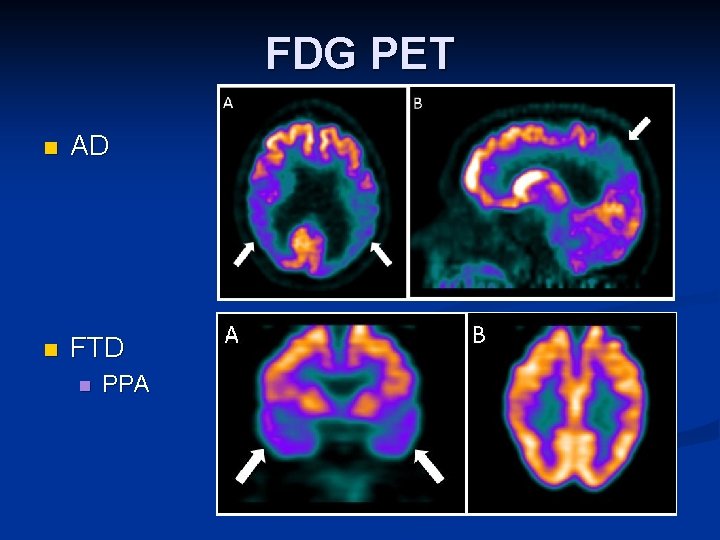
FDG PET n AD n FTD n PPA
![Amyloid Imaging Pittsburgh CompoundB PET T 1 WMRI Radiolabeled thioflavin derivative n Nmethyl11C2 4methylaminophenyl6 Amyloid Imaging: Pittsburgh Compound-B PET T 1 W-MRI Radiolabeled thioflavin derivative n [N-methyl-(11)C]2 -(4’methylaminophenyl)-6](https://slidetodoc.com/presentation_image/309139a07eedd31384e1d5b6a9816a79/image-30.jpg)
Amyloid Imaging: Pittsburgh Compound-B PET T 1 W-MRI Radiolabeled thioflavin derivative n [N-methyl-(11)C]2 -(4’methylaminophenyl)-6 hydroxybenzothiazole n Selectively binds to amyloid plaque and cerebrovascular amyloid n Significant retention seen in: n n n 90+% AD patients 60% patients with MCI 30% “normal” elderly AD n Control Pittsburgh Compound-B (PIB) PIB- PET Mathis J Med Chem 2003; 46(13) Applied Neurology, Nov. 2005 (suppl) Mosconi J Alzheimer’s Dis 2010

Commercially Available Amyloidbinding Radionucleotides n Florbetapir (Amyvid) 1 n Marketed in US by Eli Lilly Approved by FDA, not covered by CMS for routine use 2 n Half life 110 minutes n n Additional FDA-approved radionucleotides 3 n n Florbetaben (Neuraceq, Piramal Imaging) Flutemetamol (Vizamyl, GE Healthcare) 1: Florbetapir, package insert 2: CMS Memo (CAG-00431 N) 3: Alzforum, downloaded 8. 5. 14

But… Aẞ burden as assessed by positron emission tomography(PET) does not strongly correlate with cognitive impairment in AD patients

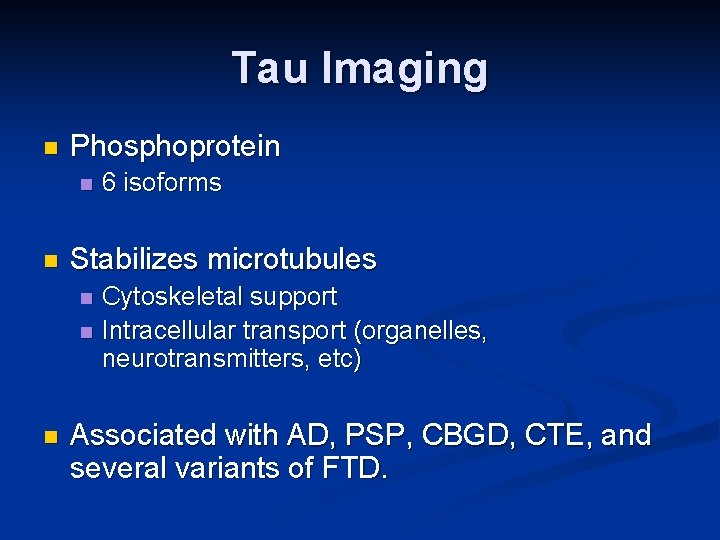
Tau Imaging n Human postmortem studies have shown that it is the density of NFTs and not of Aẞ insoluble plaques that strongly correlates with neurodegeneration and cognitive deficits. Villemagne 2014

Tau Imaging n Phosphoprotein n n Stabilizes microtubules n n n 6 isoforms Cytoskeletal support Intracellular transport (organelles, neurotransmitters, etc) Associated with AD, PSP, CBGD, CTE, and several variants of FTD.

AD and the Brain Neurofibrillary Tangles . NIA/ADEAR

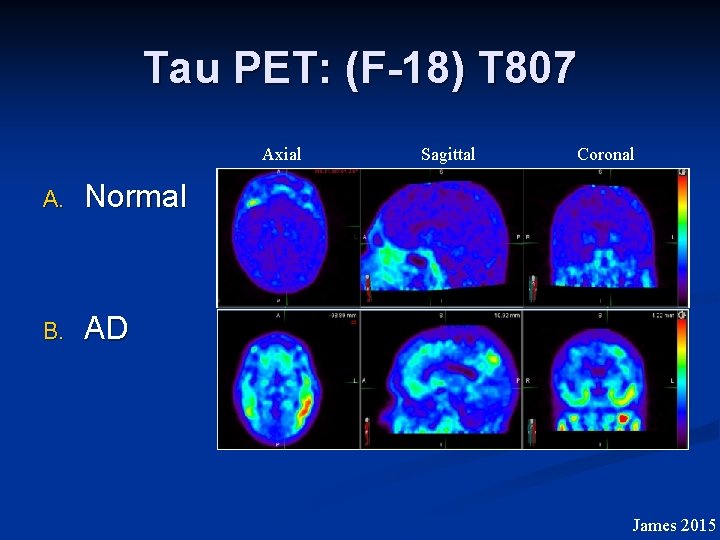
Tau PET Tracers n Ideal tau PET tracer High affinity for phosphorolated tau and neurofibrillary tangles n Weak affinity for tau monomers and amyloid n n 7+ tau tracers developed n (F-18)T 807 in phase 2 trials

Tau PET: (F-18) T 807 Axial A. Normal B. AD Sagittal Coronal James 2015

Other Degenerative Dementias Frontotemporal dementia (FTD) n Movement Disorders n Prion Disorders n Vascular dementia (VAD) n

Frontotemporal Degeneration (FTD)

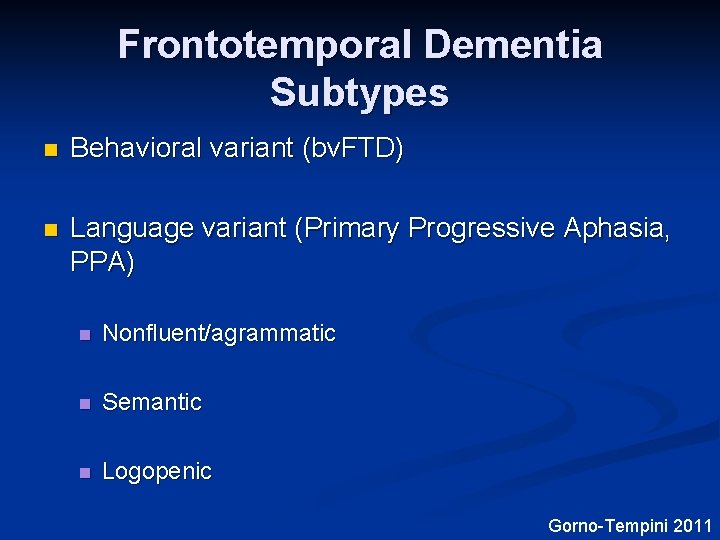
Frontotemporal Dementia Subtypes n Behavioral variant (bv. FTD) n Language variant (Primary Progressive Aphasia, PPA) n Nonfluent/agrammatic n Semantic n Logopenic Gorno-Tempini 2011

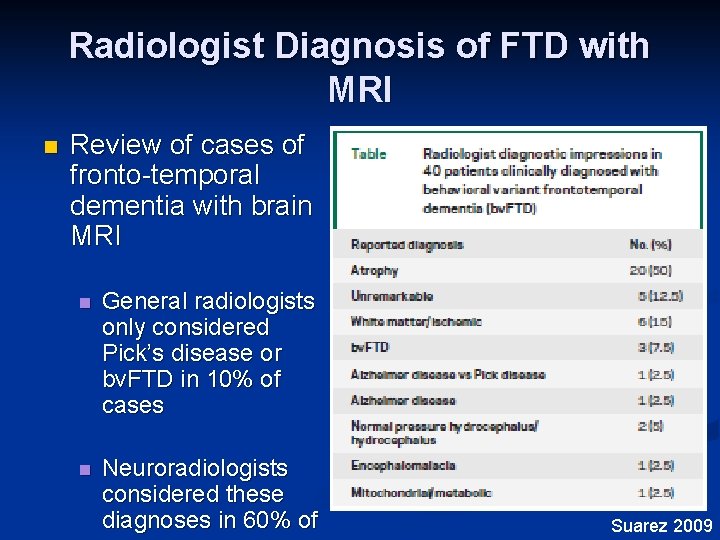
Radiologist Diagnosis of FTD with MRI n Review of cases of fronto-temporal dementia with brain MRI n General radiologists only considered Pick’s disease or bv. FTD in 10% of cases n Neuroradiologists considered these diagnoses in 60% of Suarez 2009

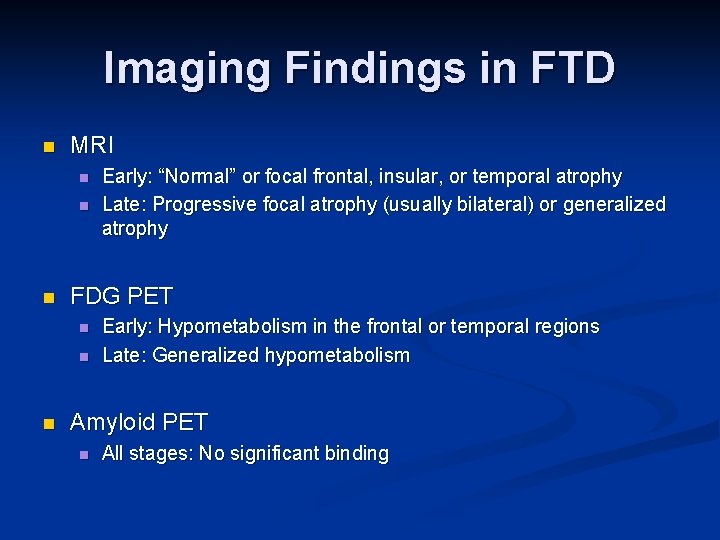
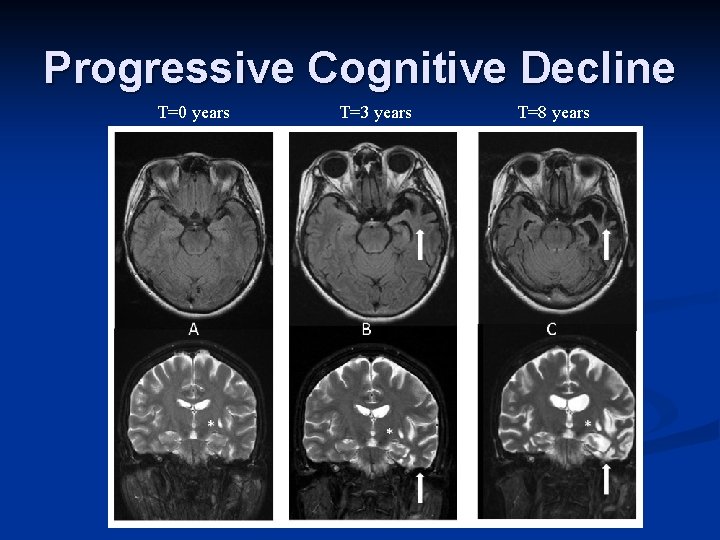
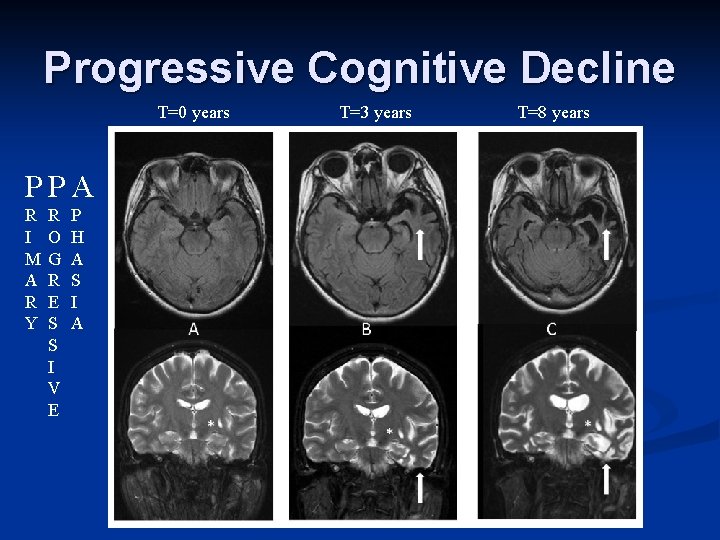
Imaging Findings in FTD n MRI n n n FDG PET n n n Early: “Normal” or focal frontal, insular, or temporal atrophy Late: Progressive focal atrophy (usually bilateral) or generalized atrophy Early: Hypometabolism in the frontal or temporal regions Late: Generalized hypometabolism Amyloid PET n All stages: No significant binding

Progressive Cognitive Decline T=0 years T=3 years T=8 years

Progressive Cognitive Decline T=0 years PPA R I M A R Y R O G R E S S I V E P H A S I A T=3 years T=8 years

Movement Disorders

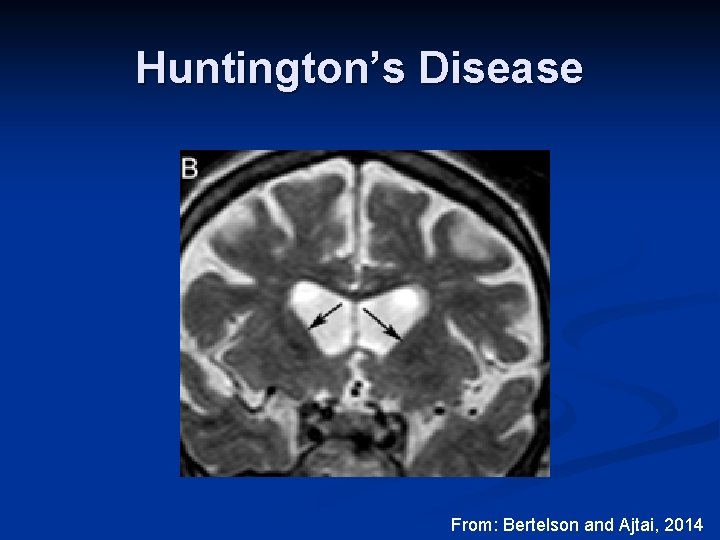
Huntington’s Disease From: Bertelson and Ajtai, 2014

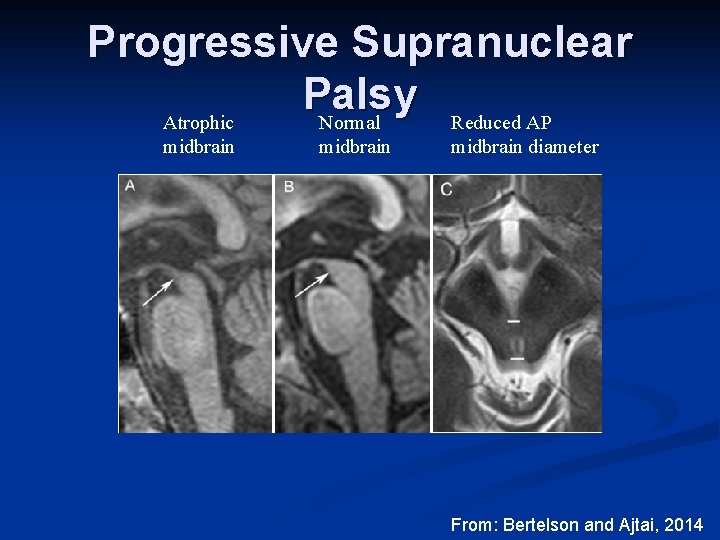
Progressive Supranuclear Palsy Reduced AP Atrophic Normal midbrain diameter From: Bertelson and Ajtai, 2014

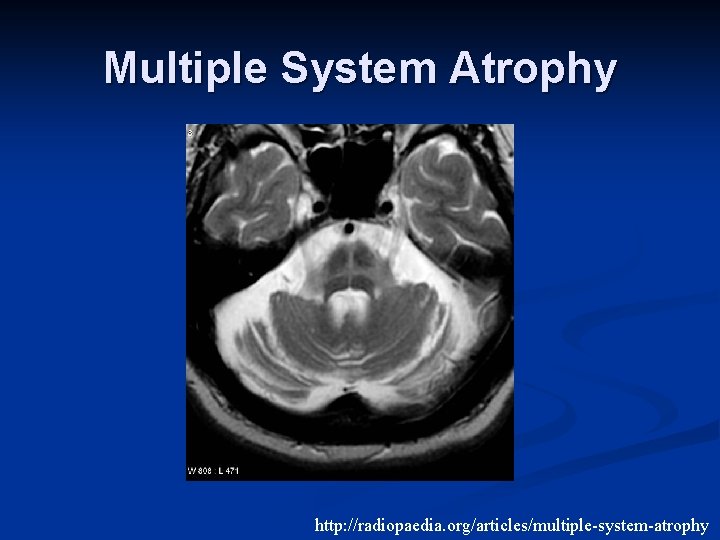
Multiple System Atrophy http: //radiopaedia. org/articles/multiple-system-atrophy

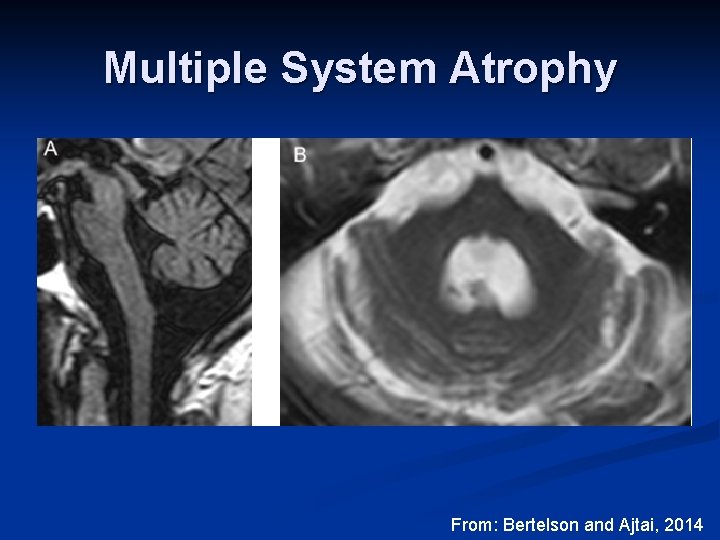
Multiple System Atrophy From: Bertelson and Ajtai, 2014

Prion Diseases

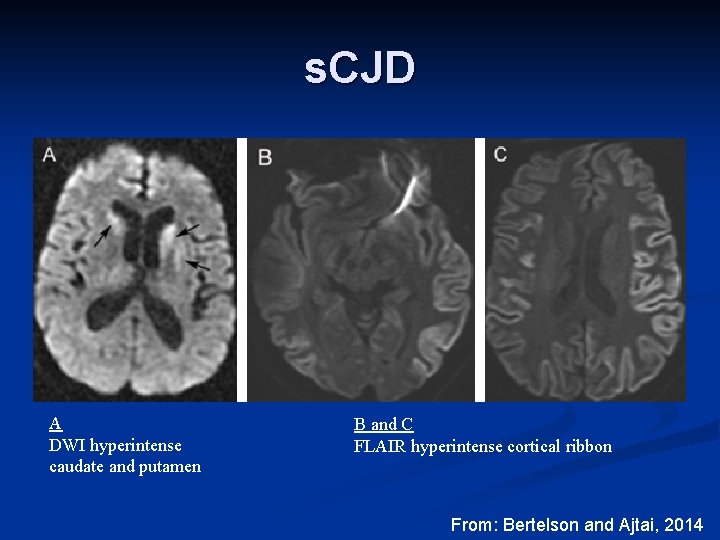
s. CJD A DWI hyperintense caudate and putamen B and C FLAIR hyperintense cortical ribbon From: Bertelson and Ajtai, 2014

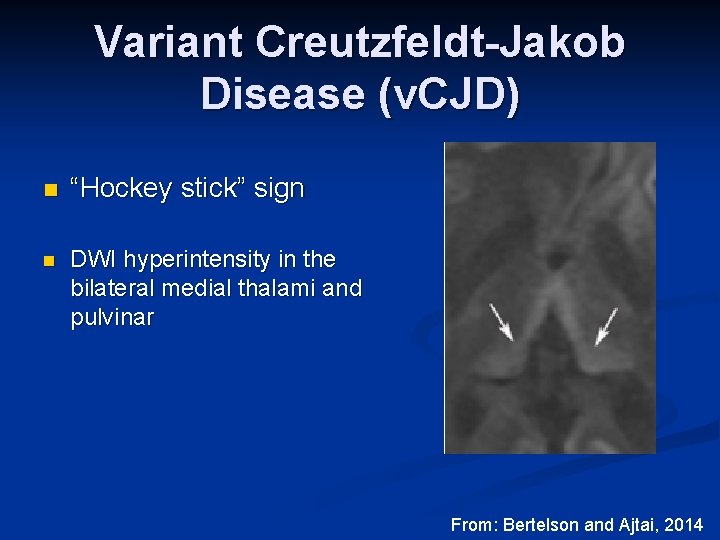
Variant Creutzfeldt-Jakob Disease (v. CJD) n “Hockey stick” sign n DWI hyperintensity in the bilateral medial thalami and pulvinar From: Bertelson and Ajtai, 2014

Vascular Dementia

Vascular Dementia (Va. D) n Definition n Dementia of a vascular cause Pohjasvaara 2000

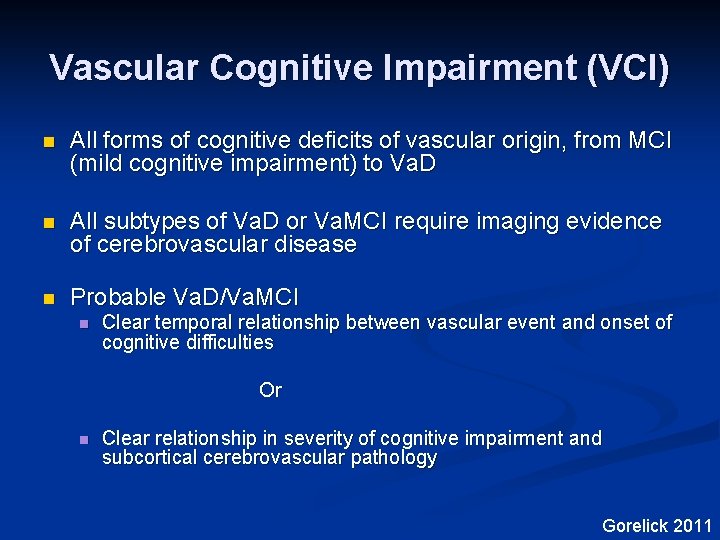
Vascular Cognitive Impairment (VCI) n All forms of cognitive deficits of vascular origin, from MCI (mild cognitive impairment) to Va. D n All subtypes of Va. D or Va. MCI require imaging evidence of cerebrovascular disease n Probable Va. D/Va. MCI n Clear temporal relationship between vascular event and onset of cognitive difficulties Or n Clear relationship in severity of cognitive impairment and subcortical cerebrovascular pathology Gorelick 2011

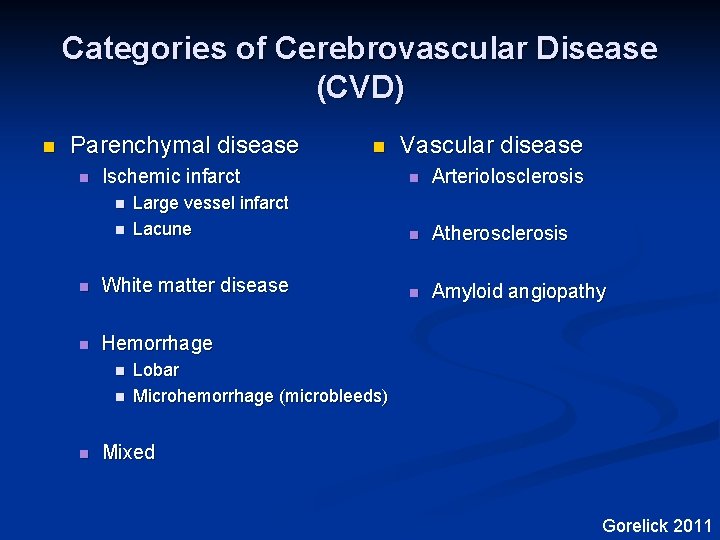
Categories of Cerebrovascular Disease (CVD) n Parenchymal disease n n Arteriolosclerosis Large vessel infarct Lacune n Atherosclerosis n White matter disease n Amyloid angiopathy n Hemorrhage n Ischemic infarct Vascular disease n n n Lobar Microhemorrhage (microbleeds) Mixed Gorelick 2011

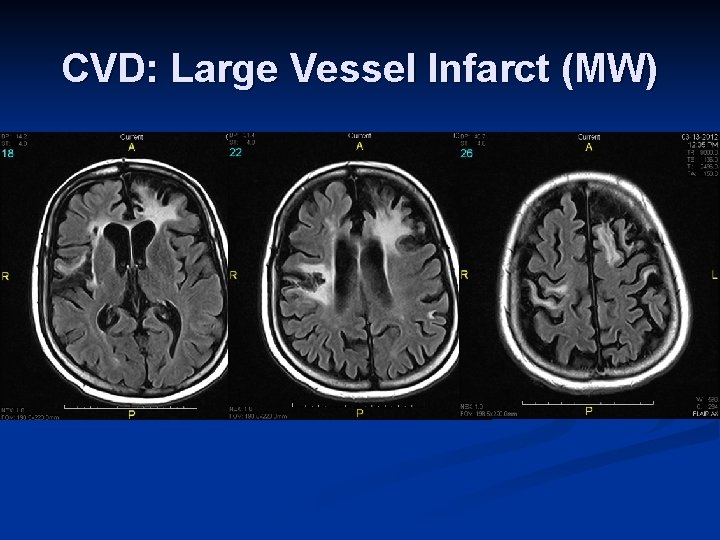
CVD: Large Vessel Infarct (MW)

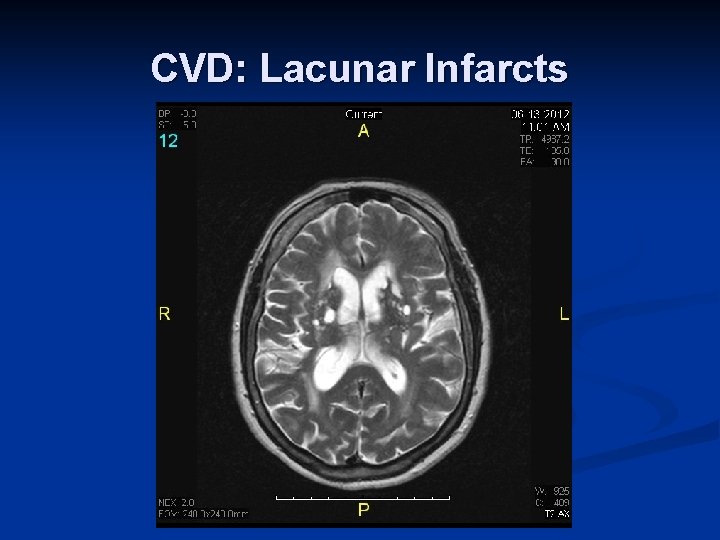
CVD: Lacunar Infarcts

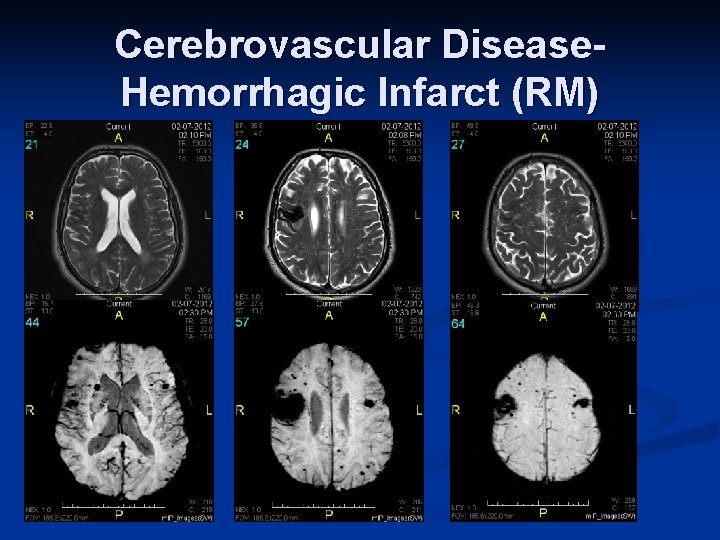
Cerebrovascular Disease. Hemorrhagic Infarct (RM)

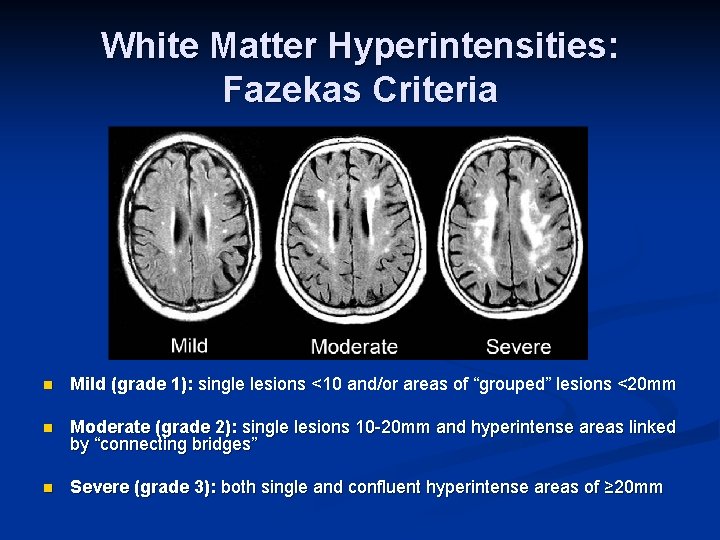
White Matter Hyperintensities: Fazekas Criteria n Mild (grade 1): single lesions <10 and/or areas of “grouped” lesions <20 mm n Moderate (grade 2): single lesions 10 -20 mm and hyperintense areas linked by “connecting bridges” n Severe (grade 3): both single and confluent hyperintense areas of ≥ 20 mm

What’s next? ? n Wider utilization of biomarkers to: n n Clarify the diagnosis Monitor response to disease modifying agents n Greater implementation of multimodal imaging n Limitations n n n Cost Access to advanced imaging Inadequacy of response to disease modifying agents

Thank You

References n Villemagne, VL et al. Tau imaging: early progress and future directions. Lancet Neurology 14 : 1(2015) 114 -24. n Villemagne VL and Okamura N. In vivo tau imaging: Obstacles and progress. Alzheimer’s and Dementia 10(2014) S 254 -64. n James OG et al. PET imaging of tau pathology in Alzheimer’s tauopathies. Frontiers in Neurology 6(2015): 1 -4. n Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, et al. Towards defining the preclinical stages

References n Alter, M et al (Quality Standards Subcommittee of the AAN). Practice parameter for diagnosis and evaluation of dementia. Neurology 1994; 44: 2203 -6. n Knopman DS et al. Practice Parameter: diagnosis of dementia (an evidence-based review). Neurology 2001; 56: 1143 -53. n Hejl A et al. Potentially reversible conditions in 1000 consecutive memory clinic patients. J Neurosurg Psychiatry 2002; 73(4): 390 -4. n Jack CR Jr, Albert M, Knopman D, Mc. Khann G, Sperling R, Carrillo M, et al. Introduction to the revised criteria for the diagnosis of Alzheimer’s disease: National Institute on Aging and the Alzheimer’s Association workgroup.

References n Mc. Khann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR Jr, Kawas CH, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging and the Alzheimer’s Association workgroup. Alzheimers Dement 2011; 7: 26369. n Albert M, De. Kosky ST, Dickson D, Dubois B, Feldman H, Fox NC, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: report of the National Institute on Aging and the Alzheimer’s Association workgroup. Alzheimers Dement 2011; 7: 270 -9. n Kalkonde YV et al. Difference between clinical subspecialties in the outpatient evaluation and treatment of dementia in an academic medical center. Dement

References n Borghesani PR et al. Neuroimaging in the clinical diagnosis of dementia: observations from a memory disorders clinic. Journal of the American Geriatrics Society 2010; 58(8): 1453 -8. n Heister D et al. Predicting MCI outcome with clinically available MRI and CSF biomarkers. Neurology 2011; 77: 1619 -28. n Dickerson BC et al. Alzheimer-signature MRI biomarker predicts AD dementia in cognitively normal adults. Neurology 2011; 76: 1395 -402. n Dickerson BC et al. MRI cortical thickness biomarker predicts AD-like CSF and cognitive decline in normal adults. Neurology 2012; 78: 84 -90.

References n Petersen RC. New clinical criteria for the Alzheimer’s Disease Spectrum. Minnesota Medicine 2012. n Price DL et al. “Investigation of acoustic noise on 15 MRI scanners from 0. 2 T to 3 T. ” J Magn Reson Imaging 2001; 13(2): 288 -93. Li TQ and Wahlund LO. The search for neuroimaging biomarkers of Alzheimer's disease with advanced MRI techniques. Acta Radiologica. 2011; Vol. 52 (2): 211 -22. n n Gorno-Tempini ML et al. “Classification of primary progressive aphasia and its variants. ” Neurology 2011; 76: 1006 -14. n Snowden JS et al. Distinct behavioural profiles in frontotemporal dementia and semantic dementia. J Neurol Neurosurg Psychiatry 2001; 70: 323 -32.

References n Rabinovici GD, Rosen HJ, etal. Amyloid vs. FDG-PET in the differential diagnosis of AD and FTLD. Neurology 2011; 77: 2034 -42. n Suárez J et al. Characterizing radiology reports in patients with frontotemporal dementia. Neurology 2009; 73(13): 1073 -4. n Kerholz K et al. “Positron emission tomography imaging in dementia. ” British Journal of Radiology 2007; 80: S 1607. n Mosconi L et al. “Pre-clinical detection of Alzheimer’s disease using FDG-PET with or without amyloid imaging. ” J Alzheimers Dis. 2010; 20(3): 843 -54.