IIon Exchange Separations Complexometric Titrations Dr Prem D







- Slides: 20

I-Ion Exchange Separations: Complexometric Titrations Dr. Prem D. Sattsangi Copyright © 2009

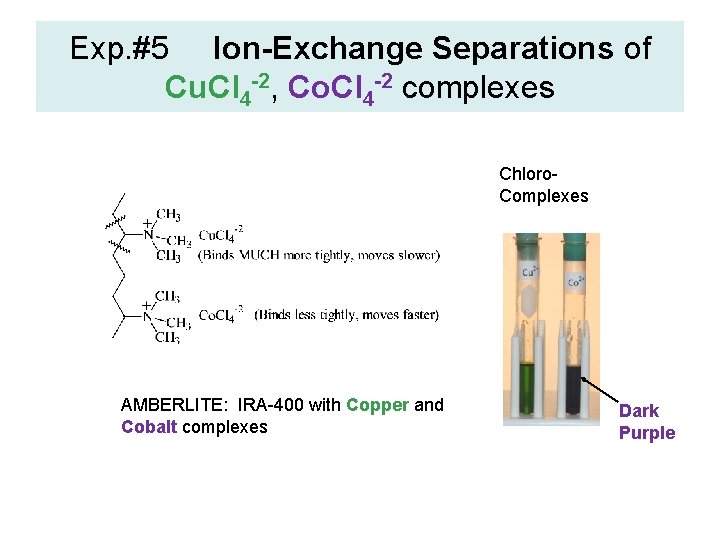
Exp. #5 Ion-Exchange Separations of Cu. Cl 4 -2, Co. Cl 4 -2 complexes Chloro. Complexes AMBERLITE: IRA-400 with Copper and Cobalt complexes Dark Purple

#2 -Chloro-Complexes, U. K. #9, Volumetric Flasks 1 -3 Chloro. Complexes U. K. Mixture Labeled

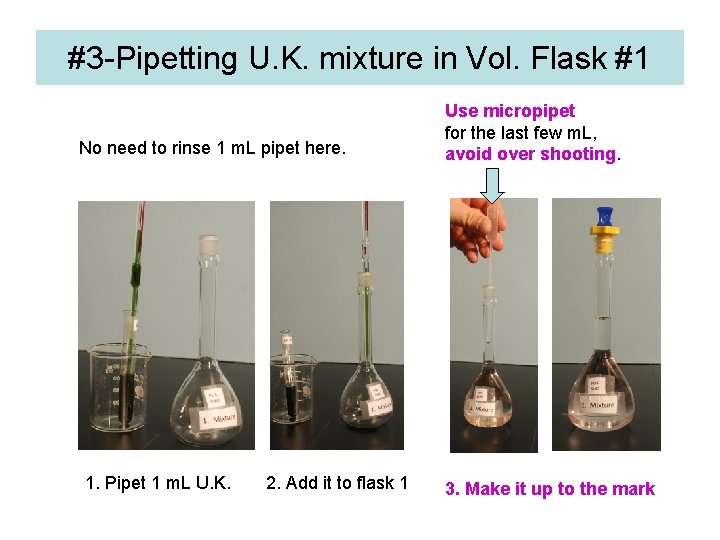
#3 -Pipetting U. K. mixture in Vol. Flask #1 No need to rinse 1 m. L pipet here. 1. Pipet 1 m. L U. K. 2. Add it to flask 1 Use micropipet for the last few m. L, avoid over shooting. 3. Make it up to the mark

#4 -Get Ready with Resin Column 1. 2. 3. 4. Resin column is prepared in 3 M HCl. Clamp it securely to a Buret stand. Place a 50 m. L Beaker underneath. Take 50 m. L, 3 M HCl in a 50 m. L Graduated Cylinder and a micropipet.

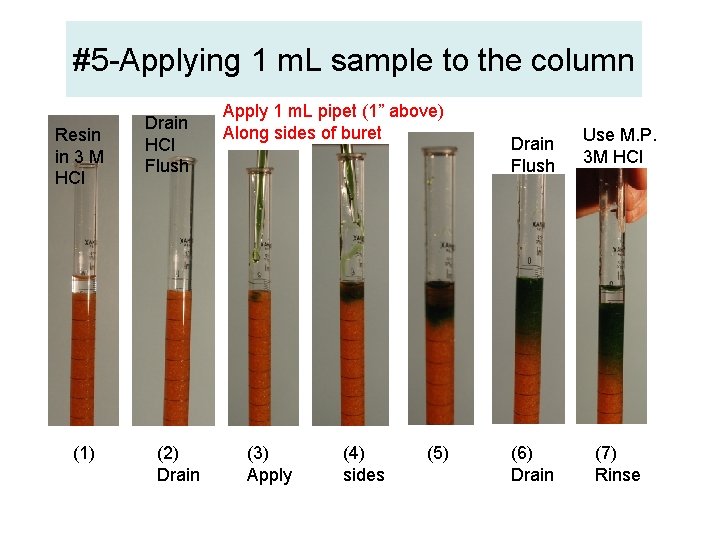
#5 -Applying 1 m. L sample to the column Resin in 3 M HCl (1) Drain HCl Flush (2) Drain Apply 1 m. L pipet (1” above) Along sides of buret (3) Apply (4) sides (5) Drain Flush (6) Drain Use M. P. 3 M HCl (7) Rinse

#6 -Insert a small wad of cotton on the top. Elute with 3 M HCl. Add 3 M HCl. Do NOT Over-fill. Keep watch on the Migrating Band. It grows fainter as it migrates. Chloro-complexes are changing to aquo- complexes. Notice the Faint Greenish band 11 -14 m. L region

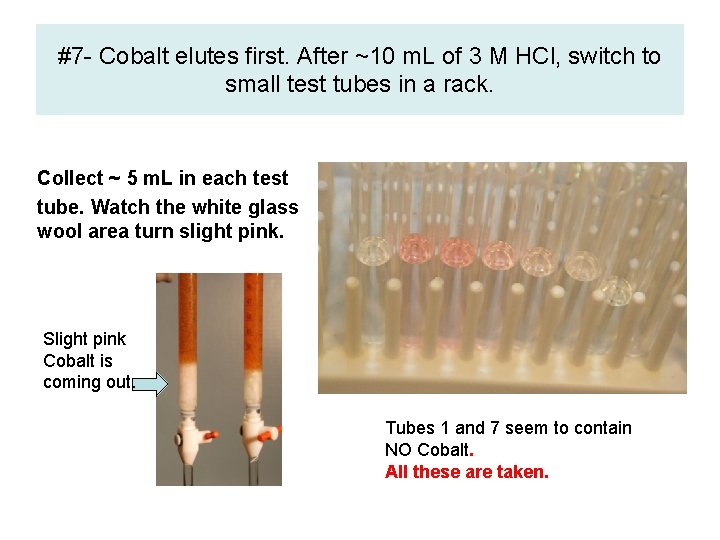
#7 - Cobalt elutes first. After ~10 m. L of 3 M HCl, switch to small test tubes in a rack. Collect ~ 5 m. L in each test tube. Watch the white glass wool area turn slight pink. Slight pink Cobalt is coming out. Tubes 1 and 7 seem to contain NO Cobalt. All these are taken.

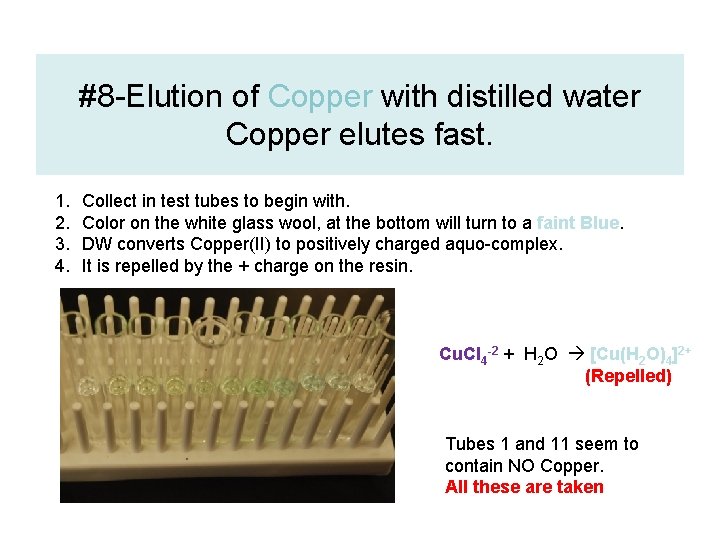
#8 -Elution of Copper with distilled water Copper elutes fast. 1. 2. 3. 4. Collect in test tubes to begin with. Color on the white glass wool, at the bottom will turn to a faint Blue. DW converts Copper(II) to positively charged aquo-complex. It is repelled by the + charge on the resin. Cu. Cl 4 -2 + H 2 O [Cu(H 2 O)4]2+ (Repelled) Tubes 1 and 11 seem to contain NO Copper. All these are taken

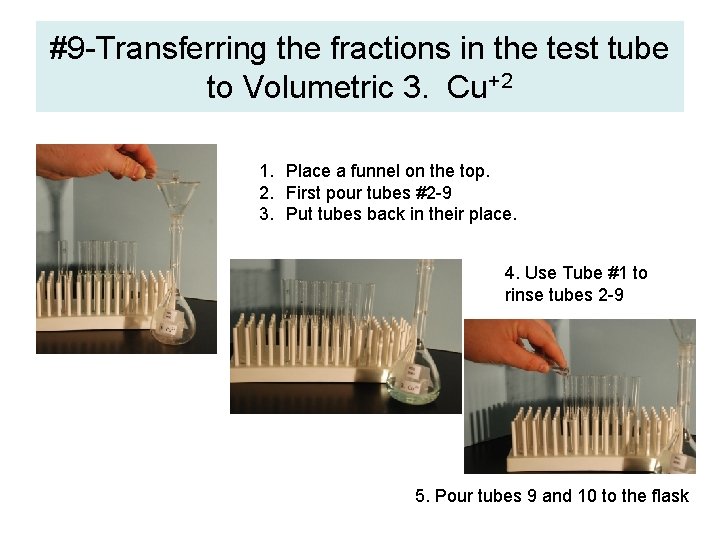
#9 -Transferring the fractions in the test tube to Volumetric 3. Cu+2 1. Place a funnel on the top. 2. First pour tubes #2 -9 3. Put tubes back in their place. 4. Use Tube #1 to rinse tubes 2 -9 5. Pour tubes 9 and 10 to the flask

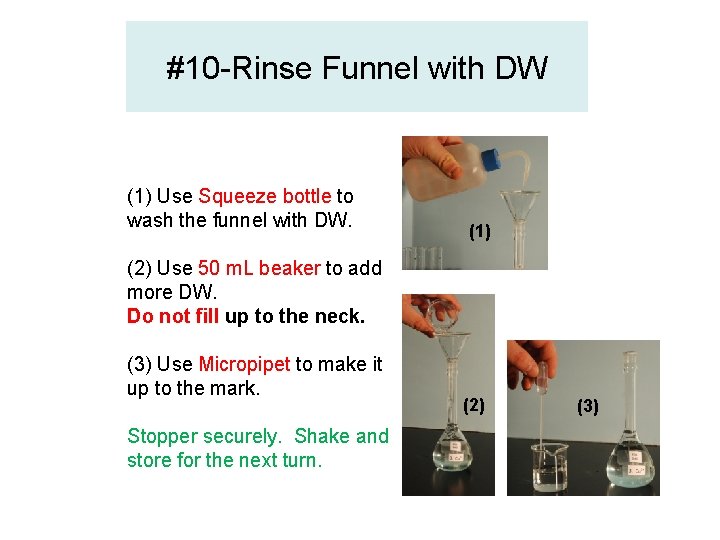
#10 -Rinse Funnel with DW (1) Use Squeeze bottle to wash the funnel with DW. (1) (2) Use 50 m. L beaker to add more DW. Do not fill up to the neck. (3) Use Micropipet to make it up to the mark. Stopper securely. Shake and store for the next turn. (2) (3)

General Advice: Economize, time, resources. Record everything directly in your note book. DO NOT WAIT FOR p. H meter. APPROXIMATE readings will do. Washing glass ware: Use cold Tap water. Flush generously. Rinse with DW, using squeeze bottle. Squirt about 5 m. L DW along sides of the flask. Drain excess on a paper towel. Weighing Murexide: Use the same weighing paper for all the weighings. Approximate 2. 2 -2. 4 g is accurate enough. After finishing, do not forget to wash generously with ordinary water the buret and the pipet, before storing.

#11 -Titration of Mixture in Vol. Flask -1 Experiment Mixture #_____ Cu 2+ (H 2 O)4 and Co 2+ (H 2 O)4 a. Pour ~20 m. L of mixture in a Beaker. Rinse the 25. 00 m. L pipet. b. Pipette 25 m. L of Mixture into 2 Erlenmeyer flasks A, and B c. Adjust p. H and make each flask ready for titration. Observations:

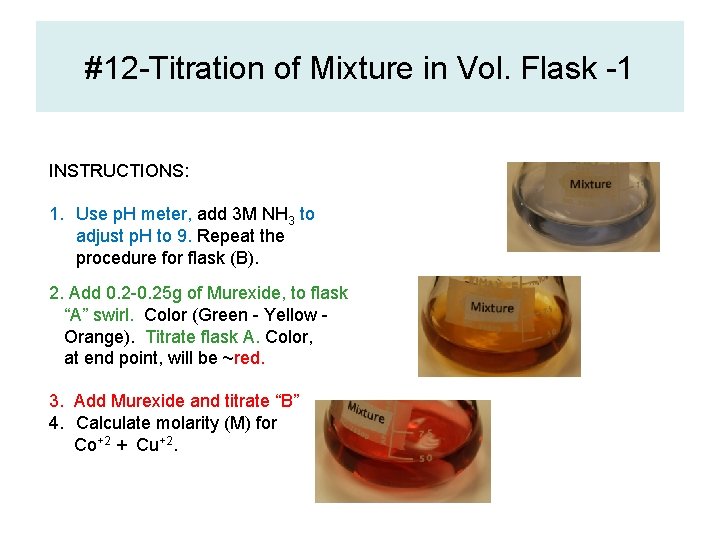
#12 -Titration of Mixture in Vol. Flask -1 INSTRUCTIONS: 1. Use p. H meter, add 3 M NH 3 to adjust p. H to 9. Repeat the procedure for flask (B). 2. Add 0. 2 -0. 25 g of Murexide, to flask “A” swirl. Color (Green - Yellow Orange). Titrate flask A. Color, at end point, will be ~red. 3. Add Murexide and titrate “B” 4. Calculate molarity (M) for Co+2 + Cu+2.

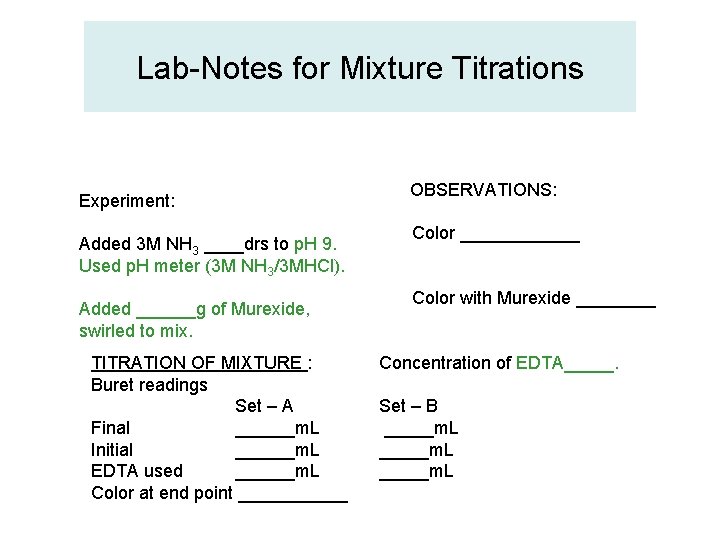
Lab-Notes for Mixture Titrations Experiment: Added 3 M NH 3 ____drs to p. H 9. Used p. H meter (3 M NH 3/3 MHCl). Added ______g of Murexide, swirled to mix. TITRATION OF MIXTURE : Buret readings Set – A Final ______m. L Initial ______m. L EDTA used ______m. L Color at end point ______ OBSERVATIONS: Color ______ Color with Murexide ____ Concentration of EDTA_____. Set – B _____m. L

#13 -Calculations One mole of EDTA reacts with 1 mol of M 2+ to form a Chelate complex. EDTA + M 2+ M(EDTA)2+complex EDTA is ~0. 01 M. Copy exact concentration of EDTA from the reagent bottle. EDTA _____M Molar concentration of Co+2 (m. L cancels) Molar concentration of Cu+2 (Show calculations same way) Molar concentration of Mixture of Co+2 and Cu+2 (Show calculations same way)

#14 -Titration of Cobalt(II) INSTRUCTIONS: (Co 2+ (H 2 O)4) 1. Rinse pipet. Take 25 m. L of Co+2 in each flask A and B. 2. Use p. H meter. Add 15 M NH 3 to p. H 8 -9 light blue/Turquoise color. 3. Add 3 M NH 3 until p. H is 9. Repeat the procedure for flask (B). 4. Add 0. 2 -0. 25 g of Murexide to flask A, swirl. Color will be orange. Titrate flasks A to a pinkish red end point. 5. Add Murexide and titrate “B” 6. Calculate molarity (M) for Co 2+.

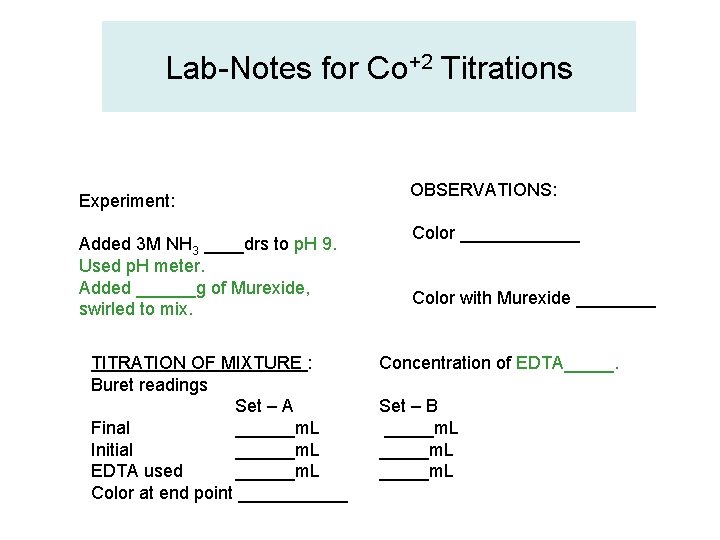
Lab-Notes for Co+2 Titrations Experiment: Added 3 M NH 3 ____drs to p. H 9. Used p. H meter. Added ______g of Murexide, swirled to mix. TITRATION OF MIXTURE : Buret readings Set – A Final ______m. L Initial ______m. L EDTA used ______m. L Color at end point ______ OBSERVATIONS: Color ______ Color with Murexide ____ Concentration of EDTA_____. Set – B _____m. L

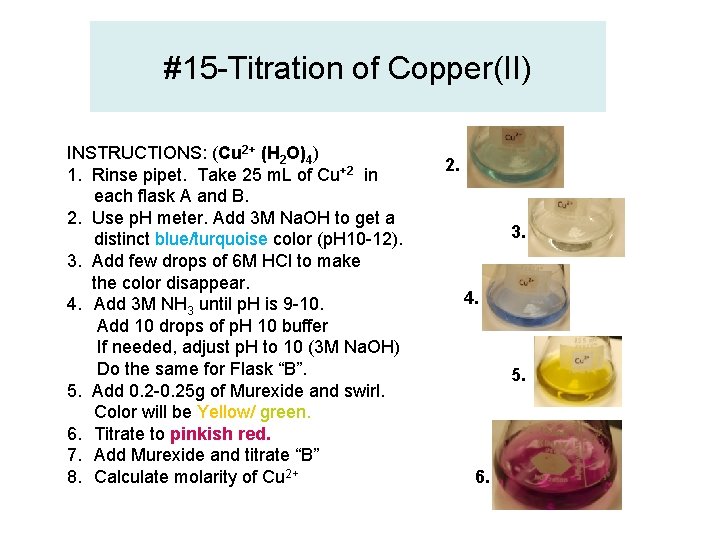
#15 -Titration of Copper(II) INSTRUCTIONS: (Cu 2+ (H 2 O)4) 1. Rinse pipet. Take 25 m. L of Cu+2 in each flask A and B. 2. Use p. H meter. Add 3 M Na. OH to get a distinct blue/turquoise color (p. H 10 -12). 3. Add few drops of 6 M HCl to make the color disappear. 4. Add 3 M NH 3 until p. H is 9 -10. Add 10 drops of p. H 10 buffer If needed, adjust p. H to 10 (3 M Na. OH) Do the same for Flask “B”. 5. Add 0. 2 -0. 25 g of Murexide and swirl. Color will be Yellow/ green. 6. Titrate to pinkish red. 7. Add Murexide and titrate “B” 8. Calculate molarity of Cu 2+ 2. 3. 4. 5. 6.

Lab-Notes for Cu+2 Titrations Experiment: Added 3 M NH 3 ____drs to p. H 9. Used p. H meter. Added ______g of Murexide, swirled to mix. TITRATION OF MIXTURE DATA: Buret readings Set – A Final ______m. L Initial ______m. L EDTA used ______m. L Color at end point ______ OBSERVATIONS: Color ______ Color with Murexide ____ Concentration of EDTA_____. Set – B _____m. L