II Patterns and forms in protein structure 2









- Slides: 39

II. Patterns and forms in protein structure 2. Patterns and forms in protein structure • Helices and sheets • The hierarchical nature of protein architecture • Structure based classification of proteins • protein folding: Intra-cellular pathogens and the survival of the flattest • Protein folding and disease: Amyloidoes, Parkinson, Huntington, Prion disease

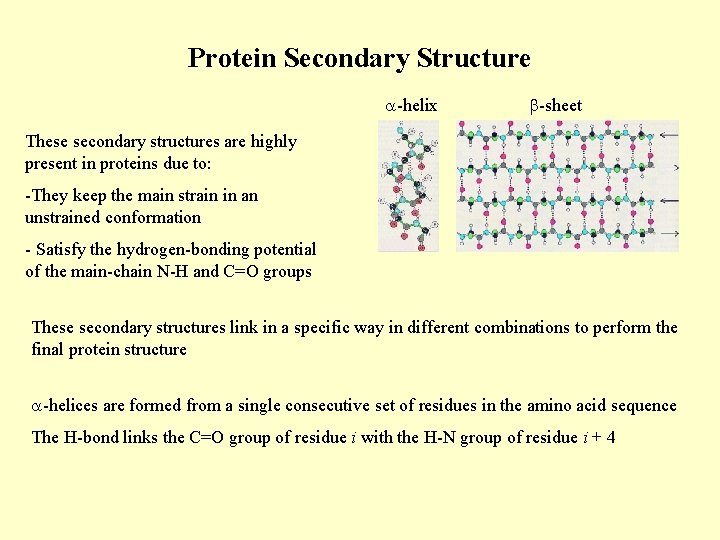
Protein Secondary Structure -helix -sheet These secondary structures are highly present in proteins due to: -They keep the main strain in an unstrained conformation - Satisfy the hydrogen-bonding potential of the main-chain N-H and C=O groups These secondary structures link in a specific way in different combinations to perform the final protein structure -helices are formed from a single consecutive set of residues in the amino acid sequence The H-bond links the C=O group of residue i with the H-N group of residue i + 4

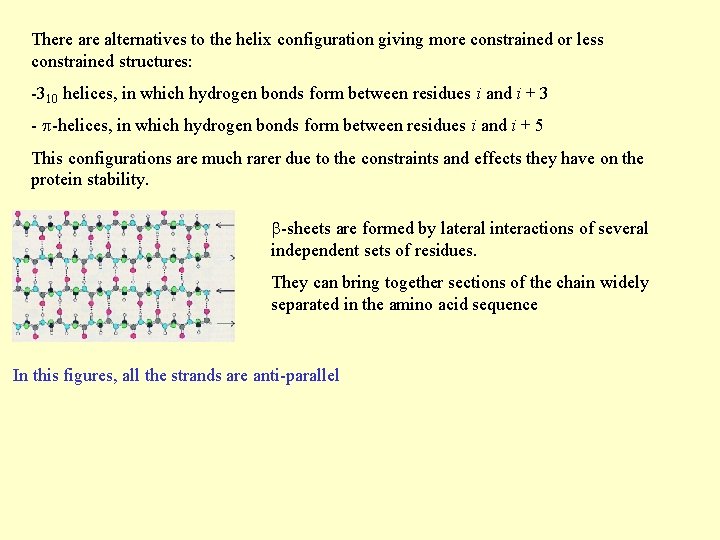
There alternatives to the helix configuration giving more constrained or less constrained structures: -310 helices, in which hydrogen bonds form between residues i and i + 3 - -helices, in which hydrogen bonds form between residues i and i + 5 This configurations are much rarer due to the constraints and effects they have on the protein stability. -sheets are formed by lateral interactions of several independent sets of residues. They can bring together sections of the chain widely separated in the amino acid sequence In this figures, all the strands are anti-parallel

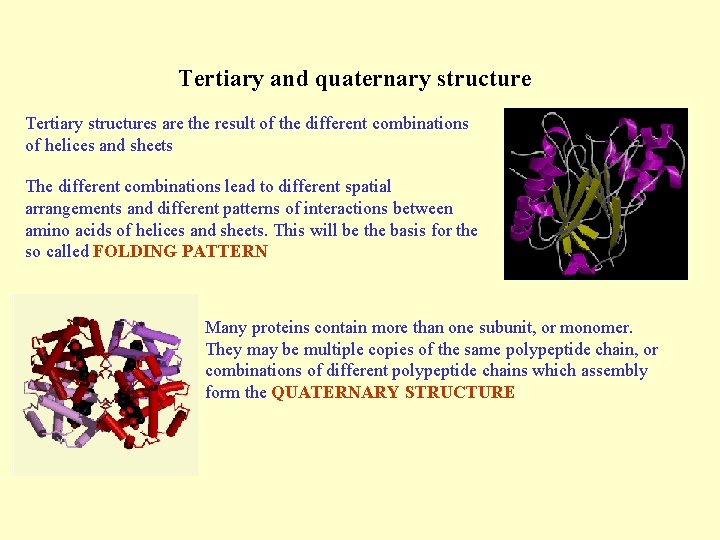
Tertiary and quaternary structure Tertiary structures are the result of the different combinations of helices and sheets The different combinations lead to different spatial arrangements and different patterns of interactions between amino acids of helices and sheets. This will be the basis for the so called FOLDING PATTERN Many proteins contain more than one subunit, or monomer. They may be multiple copies of the same polypeptide chain, or combinations of different polypeptide chains which assembly form the QUATERNARY STRUCTURE

Protein stability and denaturation The native structure of proteins can be broken up, by heating or by high concentrations of certain chemicals such as urea (DENATURATION) Denaturation destroys the secondary, tertiary and quaternary structures but leaves the polypeptide chain intact. The stability of the main chain will ensure that, ones natural conditions restored, the protein will acquire the normal productive folding conformation and thus its function. Proteins are only stable under very narrow conditions of solvent and temperatures. Breaking these conditions will break the intimate intramolecuar interactions, will change the main configurations of the backbone and will lead to non-productive conformations Giving the changeability of these conditions, the cell has developed many mechanisms to buffer these effects (Moran et al. 1996; Fares et al. 2002 a, Fares et al. 2002 b, Fares et al. 2004).

Productive protein conformation The protein conformation ensures the intra-molecular interactions that are essential forming the active sites and therefore for enabling the protein to have a biological activity. Active sites in enzymes only require 10% of the total number of amino acids in the protein. The different molecular interactions between different local secondary protein structures have the role of: - Scafolding to enable the appropriate conformation for the formation of the active site - enable conformational changes as part of the mechanism activity (Steroid Hormone receptors) - Some residues are in strained conformation playing an important role in catalysis Due to the crowded cell environment, slow-folding proteins tend to aggregate nonspecifically leading to several known diseases: Alzheimer, Prion disease The role of chaperones is essential in ensuring correct protein folding

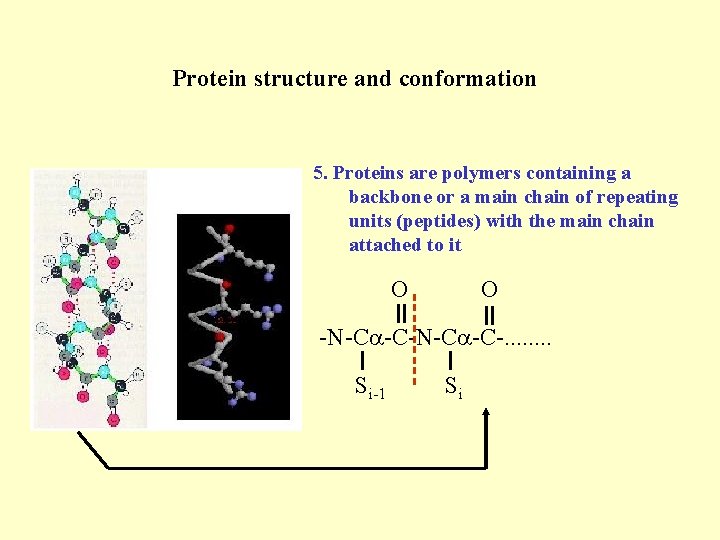
Protein structure and conformation 5. Proteins are polymers containing a backbone or a main chain of repeating units (peptides) with the main chain attached to it O O -N-C -C-. . . . Si-1 Si

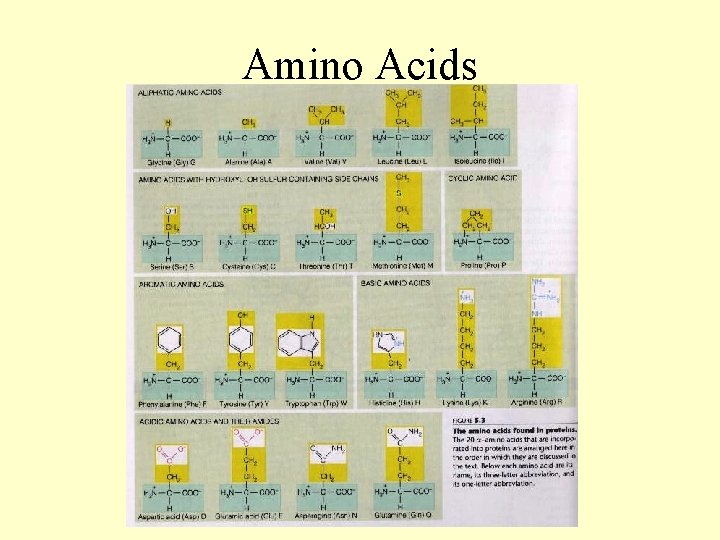
Amino Acids

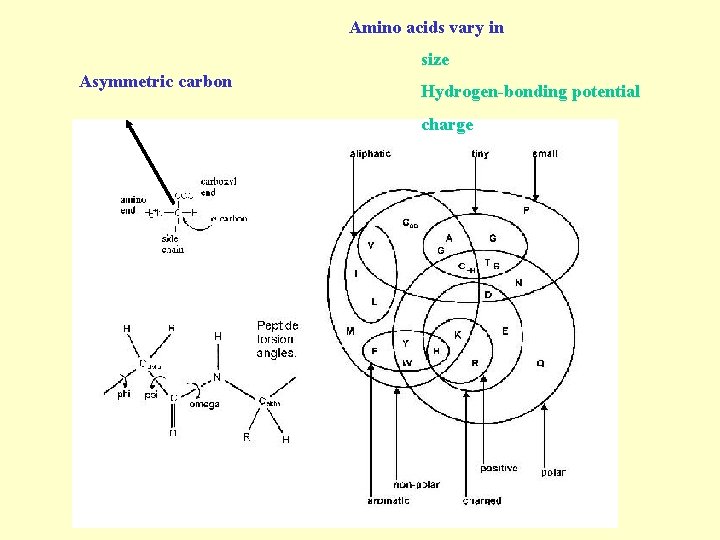
Amino acids vary in size Asymmetric carbon Hydrogen-bonding potential charge

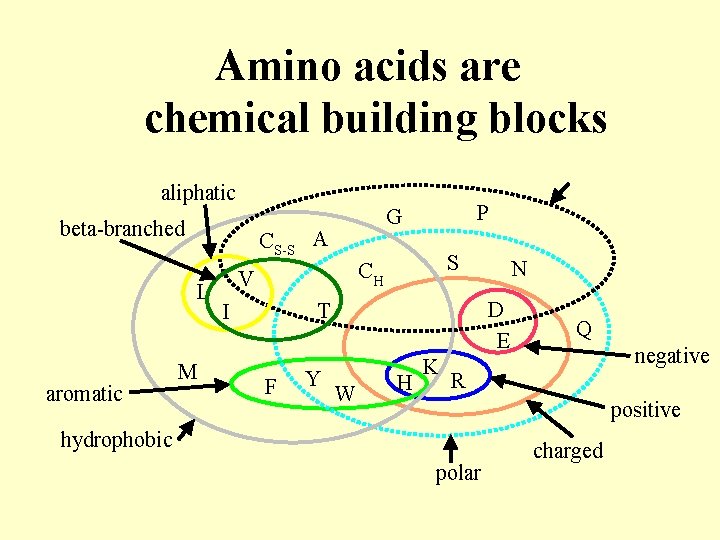
Amino acids are chemical building blocks aliphatic beta-branched CS-S A L aromatic M P G S CH V D E T I F Y N W H K Q negative R positive hydrophobic polar charged

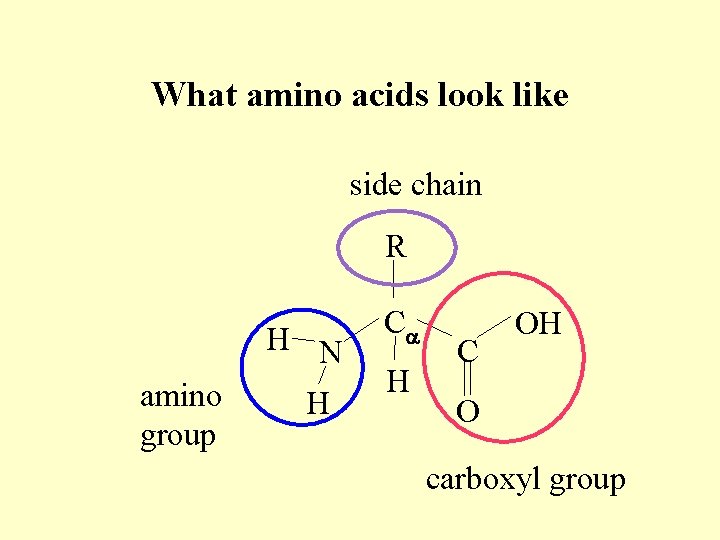
What amino acids look like side chain R H N amino group H Ca H C OH O carboxyl group

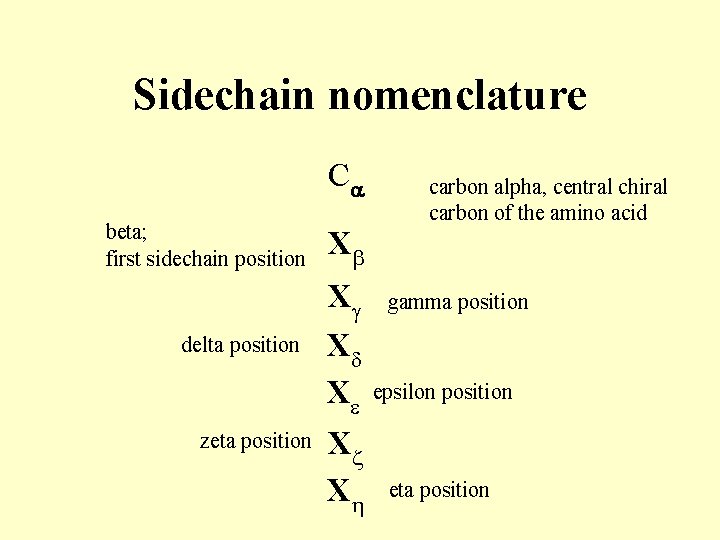
Sidechain nomenclature Ca beta; first sidechain position X delta position Xg Xd Xe zeta position Xz Xh carbon alpha, central chiral carbon of the amino acid gamma position epsilon position eta position

Small amino acids Gly: G Pro: P Ala: A Asp: D Cys: C Ser: S

Aliphatic amino acids Val: V Leu: L Ala: A Ile: I

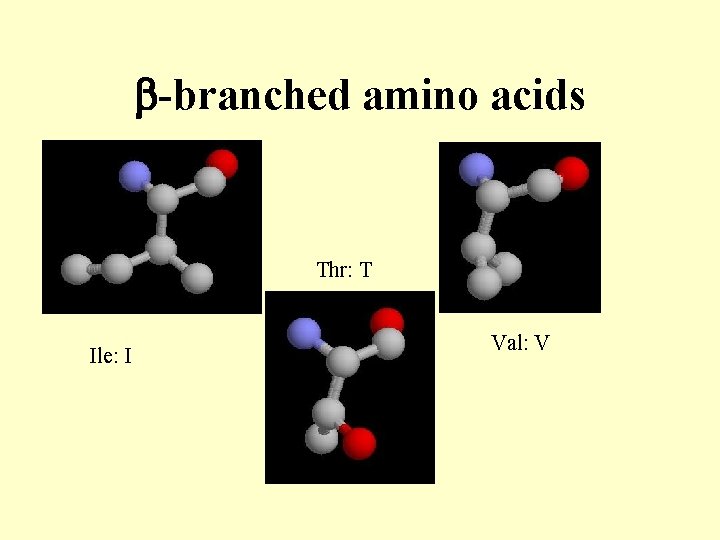
b-branched amino acids Thr: T Ile: I Val: V

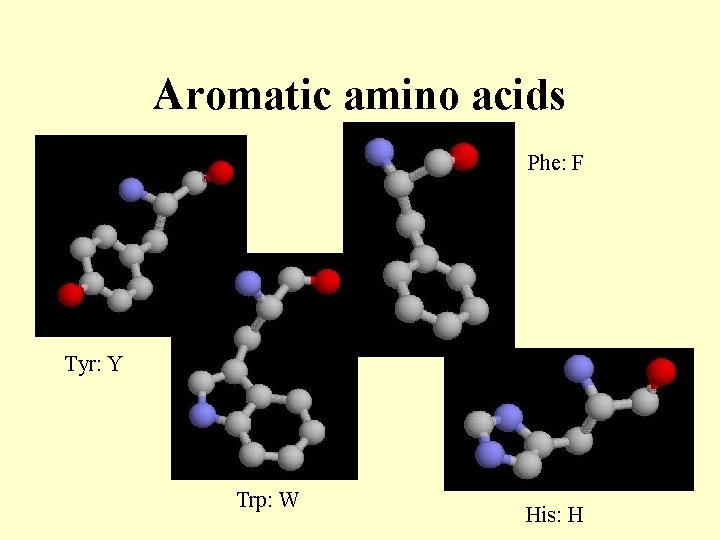
Aromatic amino acids Phe: F Tyr: Y Trp: W His: H

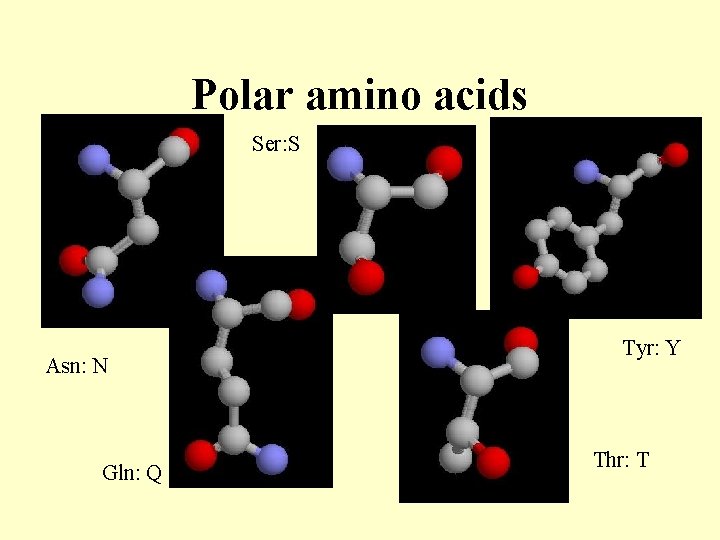
Polar amino acids Ser: S Asn: N Gln: Q Tyr: Y Thr: T

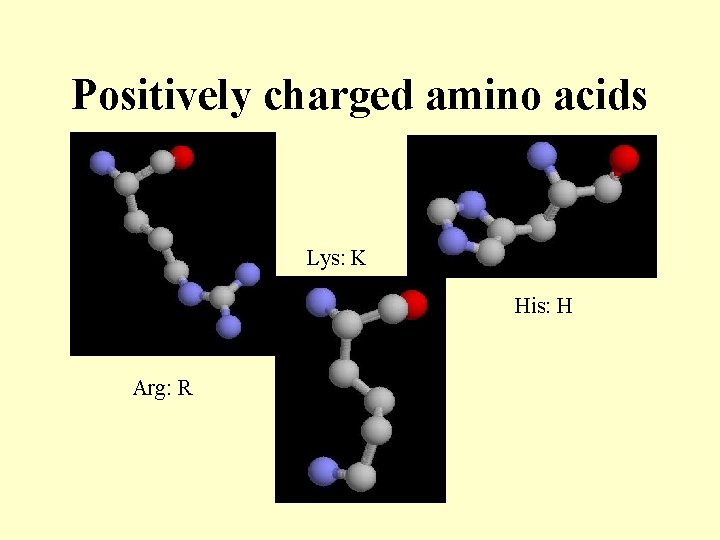
Positively charged amino acids Lys: K His: H Arg: R

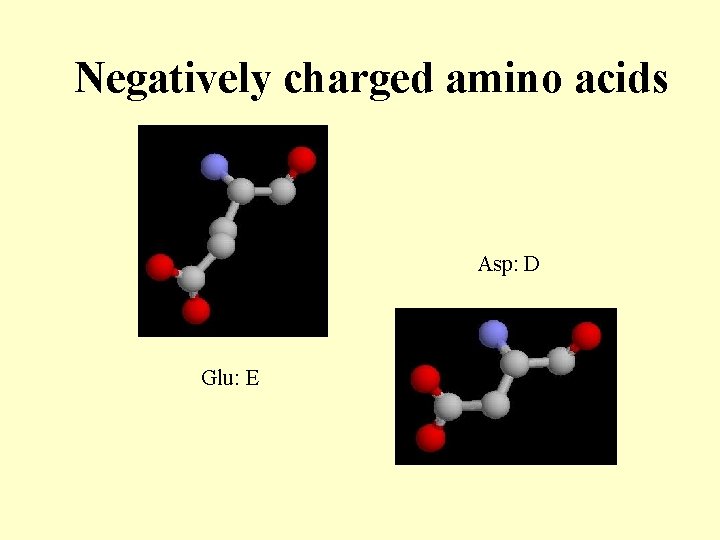
Negatively charged amino acids Asp: D Glu: E

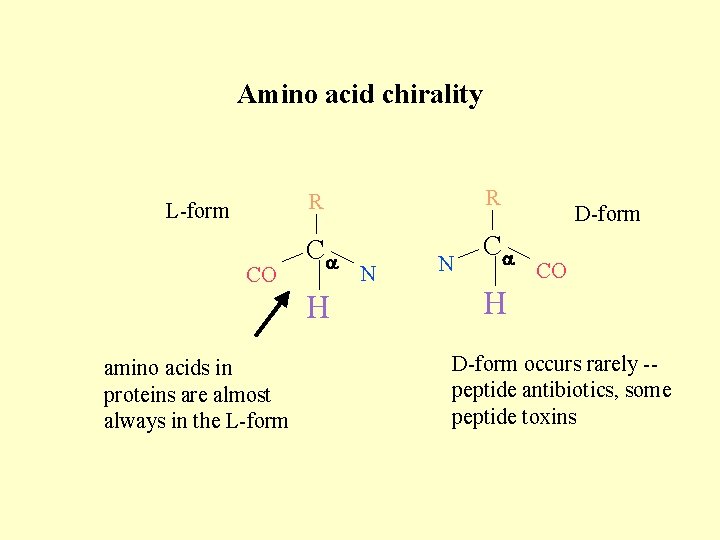
Chirality • Amino acids are not flat and two dimensional! • Groups are arranged around the central carbon atom in a tetrahedral fashion (why? ) • There are two possible ways for the groups to be arranged:

Amino acid chirality L-form CO R R Ca Ca H amino acids in proteins are almost always in the L-form N N D-form CO H D-form occurs rarely -peptide antibiotics, some peptide toxins

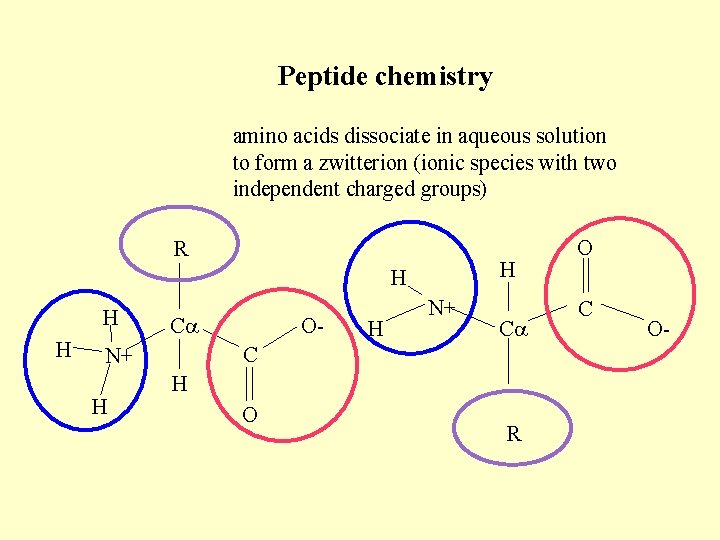
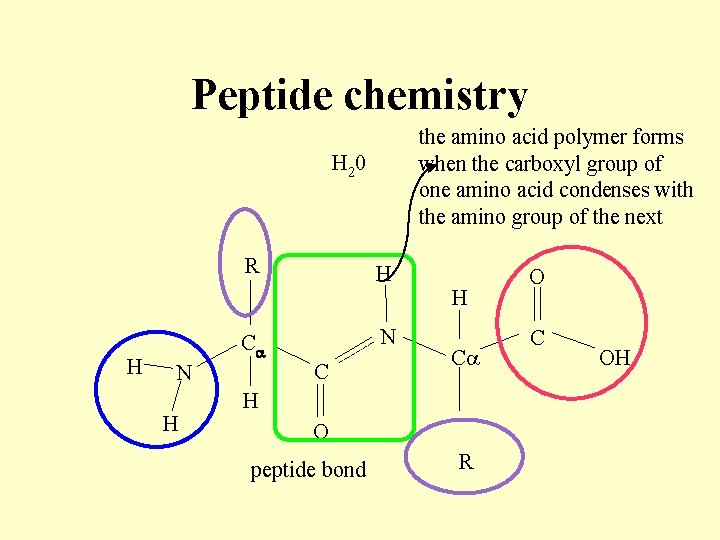
Peptide chemistry amino acids dissociate in aqueous solution to form a zwitterion (ionic species with two independent charged groups) R H H C C N+ H O- N+ H O R O C O-

Peptide chemistry the amino acid polymer forms when the carboxyl group of one amino acid condenses with the amino group of the next H 20 R H H H N H Ca N C C H O peptide bond R O C OH

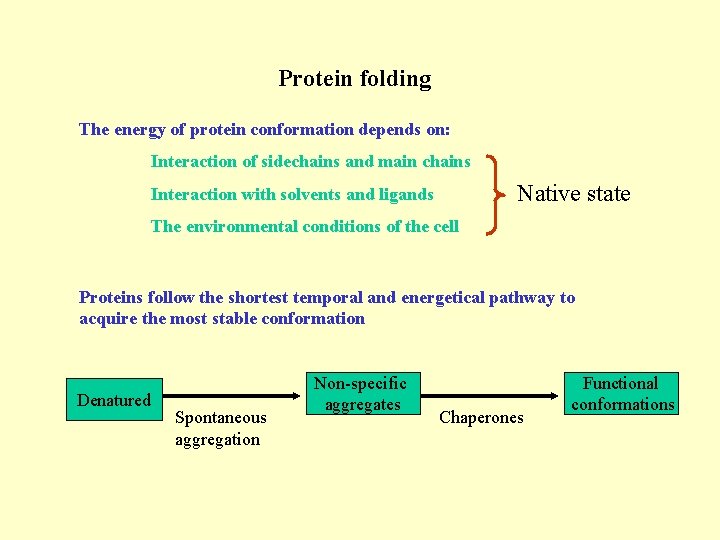
Protein folding The energy of protein conformation depends on: Interaction of sidechains and main chains Native state Interaction with solvents and ligands The environmental conditions of the cell Proteins follow the shortest temporal and energetical pathway to acquire the most stable conformation Denatured Spontaneous aggregation Non-specific aggregates Chaperones Functional conformations

Protein Folds: sequential, spatial and topological arrangement of secondary structures The Globin fold

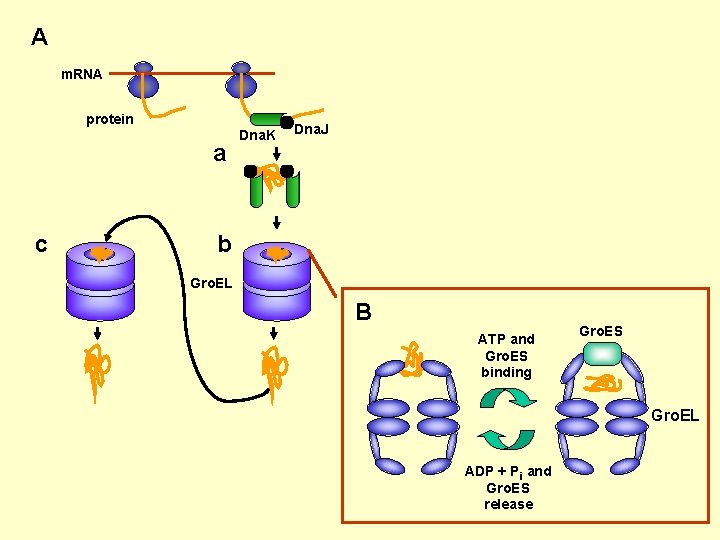
A m. RNA protein a c Dna. K Dna. J b Gro. EL B ATP and Gro. ES binding Gro. ES Gro. EL ADP + Pi and Gro. ES release

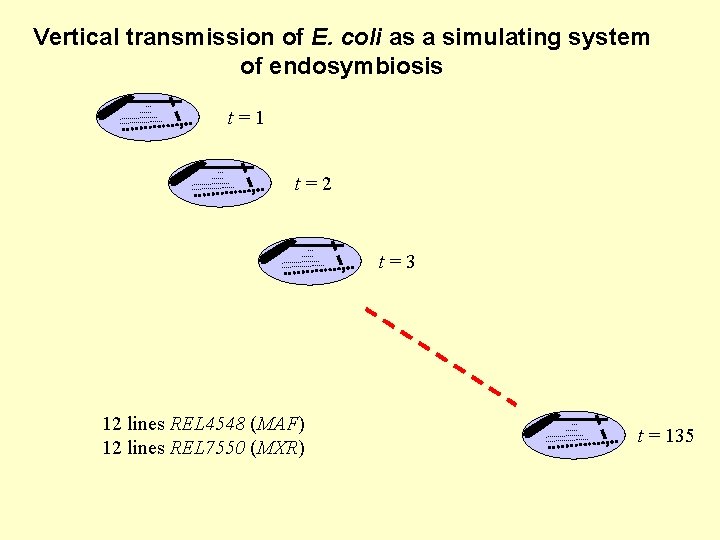
Vertical transmission of E. coli as a simulating system of endosymbiosis t=1 t=2 t=3 12 lines REL 4548 (MAF) 12 lines REL 7550 (MXR) t = 135

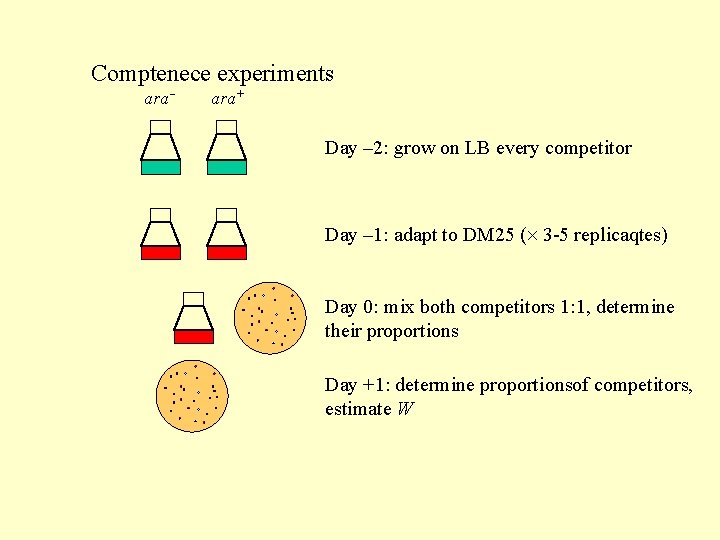
Comptenece experiments ara+ Day – 2: grow on LB every competitor Day – 1: adapt to DM 25 ( 3 -5 replicaqtes) Day 0: mix both competitors 1: 1, determine their proportions Day +1: determine proportionsof competitors, estimate W

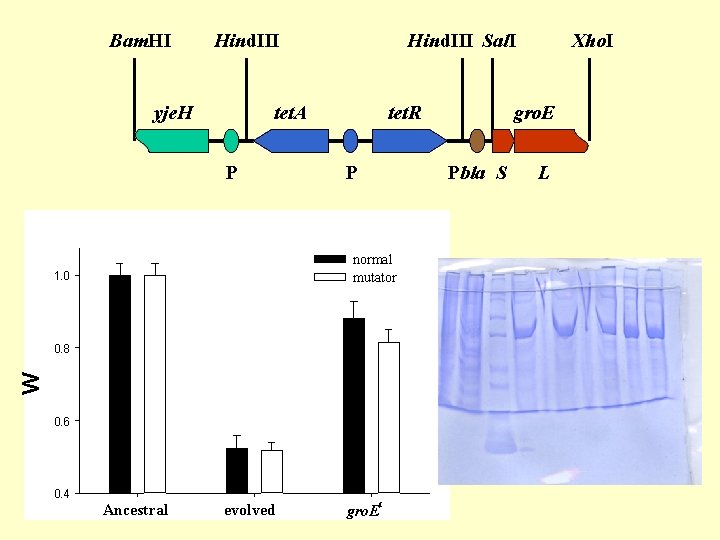
Bam. HI Hind. III yje. H Hind. III Sal. I tet. A P tet. R P 1. 0 W 0. 8 0. 6 0. 4 Ancestral evolved gro. E Pbla S normal mutator c gro. E Xho. I L

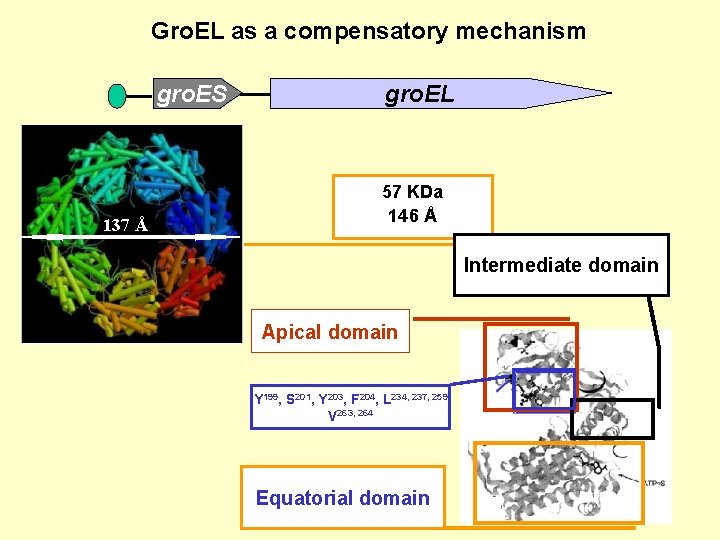
Gro. EL as a compensatory mechanism gro. ES 137 Å gro. EL 57 KDa 146 Å Intermediate domain Apical domain Y 199, S 201, Y 203, F 204, L 234, 237, 259 V 263, 264 Equatorial domain

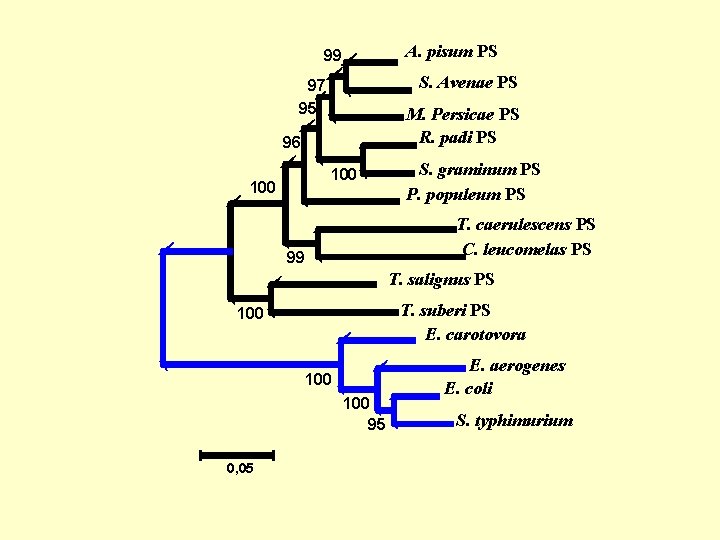
A. pisum PS 99 S. Avenae PS 97 95 M. Persicae PS R. padi PS 96 100 S. graminum PS P. populeum PS T. caerulescens PS C. leucomelas PS 99 T. salignus PS T. suberi PS E. carotovora 100 100 95 0, 05 E. aerogenes E. coli S. typhimurium

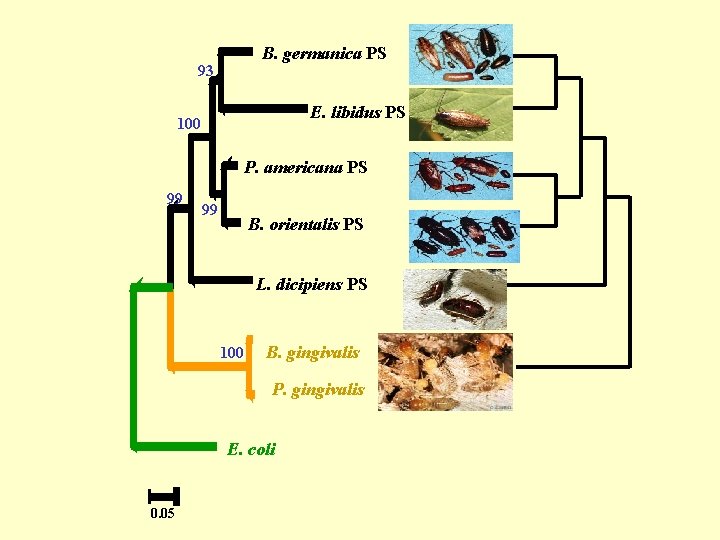
B. germanica PS 93 E. libidus PS 100 P. americana PS 99 99 B. orientalis PS L. dicipiens PS 100 B. gingivalis P. gingivalis E. coli 0. 05

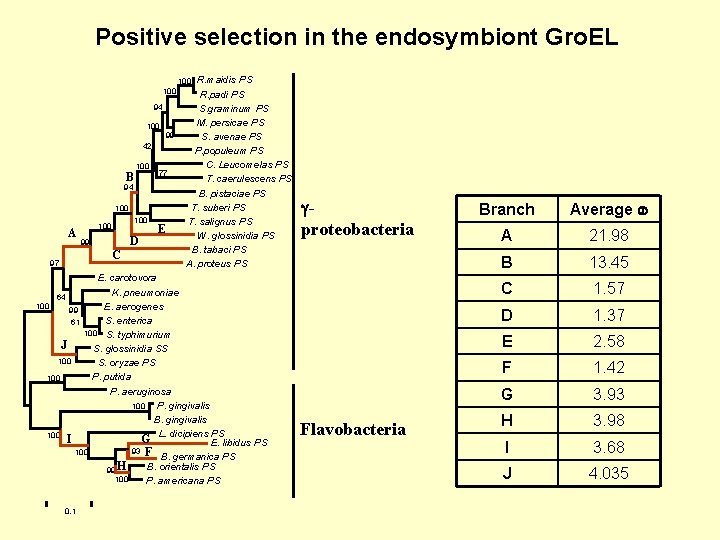
Positive selection in the endosymbiont Gro. EL R. maidis PS R. padi PS S. graminum PS M. persicae PS S. avenae PS P. populeum PS C. Leucomelas PS T. caerulescens PS B. pistaciae PS T. suberi PS T. salignus PS W. glossinidia PS B. tabaci PS A. proteus PS 100 94 100 99 42 B 100 77 94 100 A 100 D 99 C 97 100 E E. carotovora K. pneumoniae 64 100 E. aerogenes 99 S. enterica 61 100 S. typhimurium J S. glossinidia SS 100 S. oryzae PS P. putida 100 P. aeruginosa 100 P. gingivalis B. gingivalis L. dicipiens PS 100 I G E. libidus PS 93 F 100 B. germanica PS B. orientalis PS 99 H 100 P. americana PS 0. 1 proteobacteria Flavobacteria Branch Average A 21. 98 B 13. 45 C 1. 57 D 1. 37 E 2. 58 F 1. 42 G 3. 93 H 3. 98 I 3. 68 J 4. 035

Convergent adaptive evolution in Gro. EL from endosymbiotic bacteria A B

Protein structure stability and its ability and specificity to bind ligands depend on different chemical forces: Covalent bonds Hydrogen bonding

Hydrophobic effect

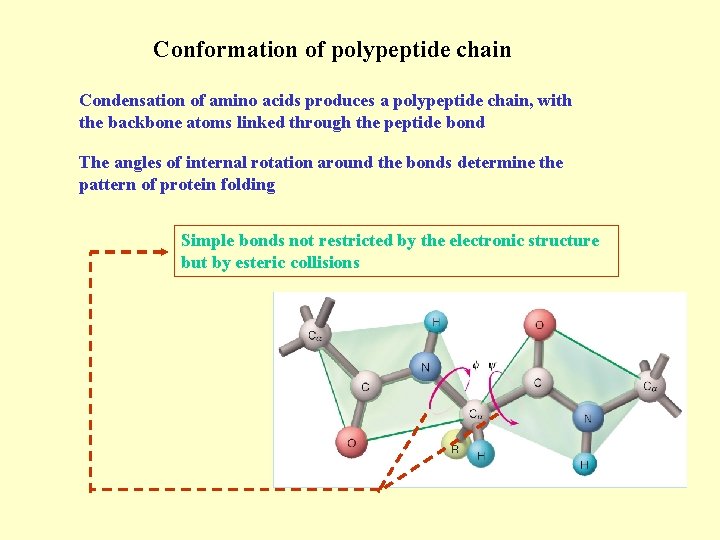
Conformation of polypeptide chain Condensation of amino acids produces a polypeptide chain, with the backbone atoms linked through the peptide bond The angles of internal rotation around the bonds determine the pattern of protein folding Simple bonds not restricted by the electronic structure but by esteric collisions

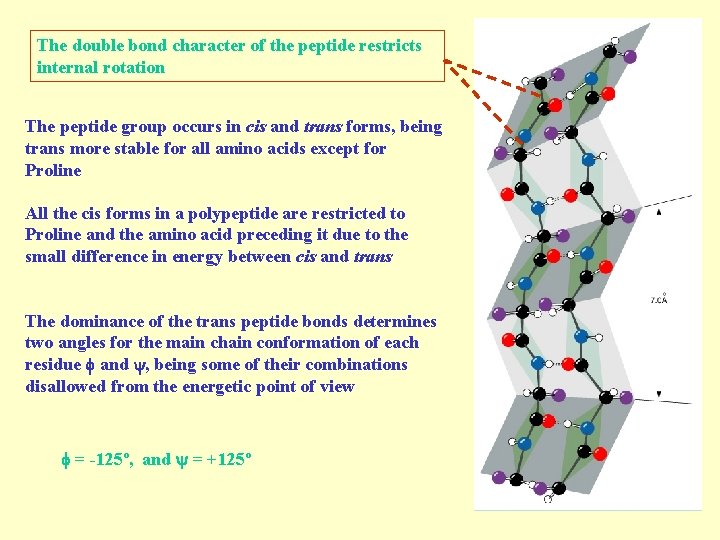
The double bond character of the peptide restricts internal rotation The peptide group occurs in cis and trans forms, being trans more stable for all amino acids except for Proline All the cis forms in a polypeptide are restricted to Proline and the amino acid preceding it due to the small difference in energy between cis and trans The dominance of the trans peptide bonds determines two angles for the main chain conformation of each residue and , being some of their combinations disallowed from the energetic point of view = -125º, and = +125º

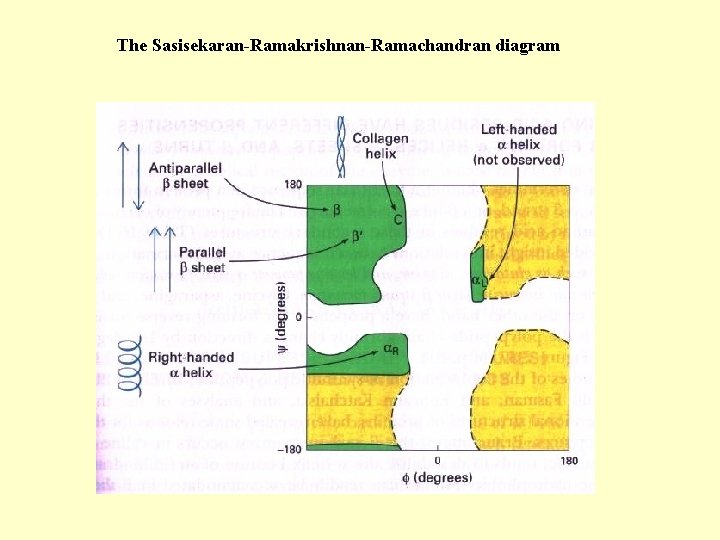
The Sasisekaran-Ramakrishnan-Ramachandran diagram