IEMR Proteins Separation purification and quantification Per Kristian



- Slides: 24

IEMR Proteins: Separation, purification and quantification Per Kristian Lunde Institute for experimental medical research www. iemr. no IEMR, Oslo Norway

IEMR Main objectives of this lecture: - Separation of proteins by gel electrophoresis - Detection of proteins in gels and membranes - Isolation of proteins from different cellular compartments by centrifugation www. iemr. no IEMR, Oslo Norway

IEMR Proteins: - Chains of aminoacids (AA, ≈20 different) - More than 50 AA in a chain is usually termed proteins, less than 50 AA = peptides (Titin: more than 34000 AA => Mw > 3800 k. Da) - Different types of proteins: Structural, membrane, soluble…. . - A protein may consist of one or several AA chains Ryanodine receptor, Efremov et al, Nature 2015, 517, 39 www. iemr. no IEMR, Oslo Norway

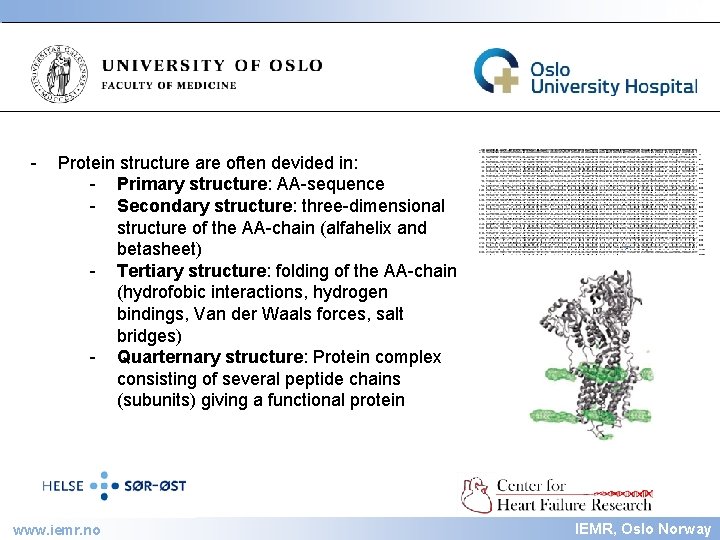
IEMR - Protein structure are often devided in: - Primary structure: AA-sequence - Secondary structure: three-dimensional structure of the AA-chain (alfahelix and betasheet) - Tertiary structure: folding of the AA-chain (hydrofobic interactions, hydrogen bindings, Van der Waals forces, salt bridges) - Quarternary structure: Protein complex consisting of several peptide chains (subunits) giving a functional protein www. iemr. no IEMR, Oslo Norway

IEMR Separation of proteins: - Electrophoresis Sentrifugation/sedimentation Precipitation www. iemr. no IEMR, Oslo Norway

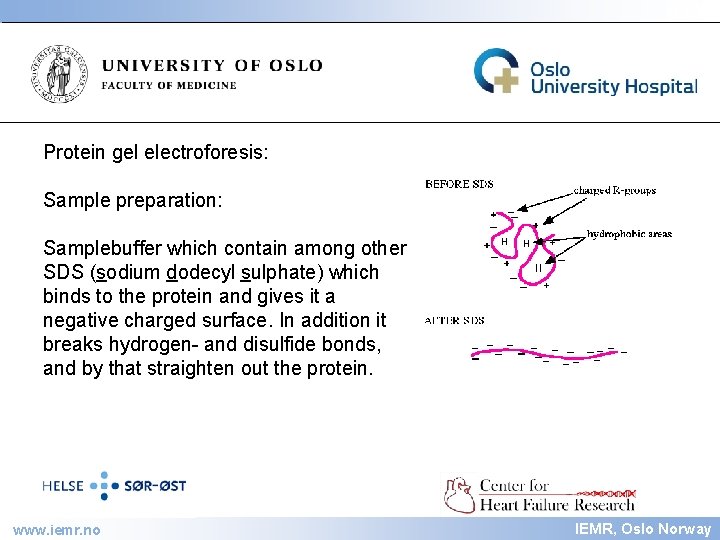
IEMR Protein gel electroforesis: Sample preparation: Samplebuffer which contain among other SDS (sodium dodecyl sulphate) which binds to the protein and gives it a negative charged surface. In addition it breaks hydrogen- and disulfide bonds, and by that straighten out the protein. www. iemr. no IEMR, Oslo Norway

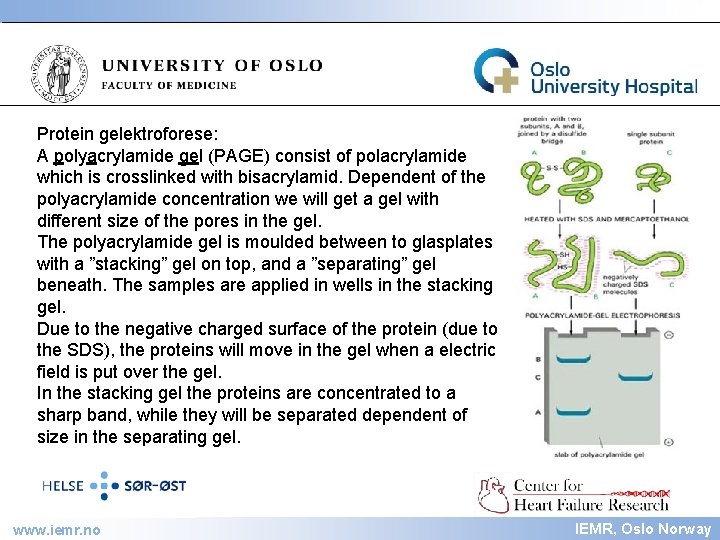
IEMR Protein gelektroforese: A polyacrylamide gel (PAGE) consist of polacrylamide which is crosslinked with bisacrylamid. Dependent of the polyacrylamide concentration we will get a gel with different size of the pores in the gel. The polyacrylamide gel is moulded between to glasplates with a ”stacking” gel on top, and a ”separating” gel beneath. The samples are applied in wells in the stacking gel. Due to the negative charged surface of the protein (due to the SDS), the proteins will move in the gel when a electric field is put over the gel. In the stacking gel the proteins are concentrated to a sharp band, while they will be separated dependent of size in the separating gel. www. iemr. no IEMR, Oslo Norway

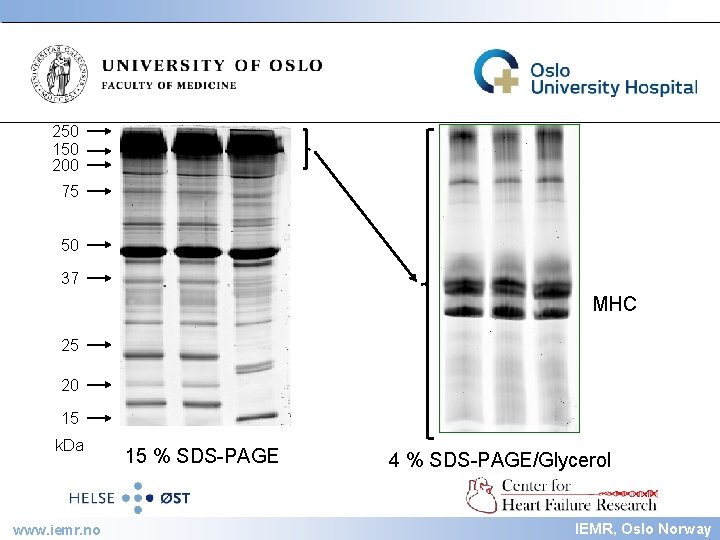
IEMR 250 150 200 75 50 37 MHC 25 20 15 k. Da www. iemr. no 15 % SDS-PAGE 4 % SDS-PAGE/Glycerol IEMR, Oslo Norway

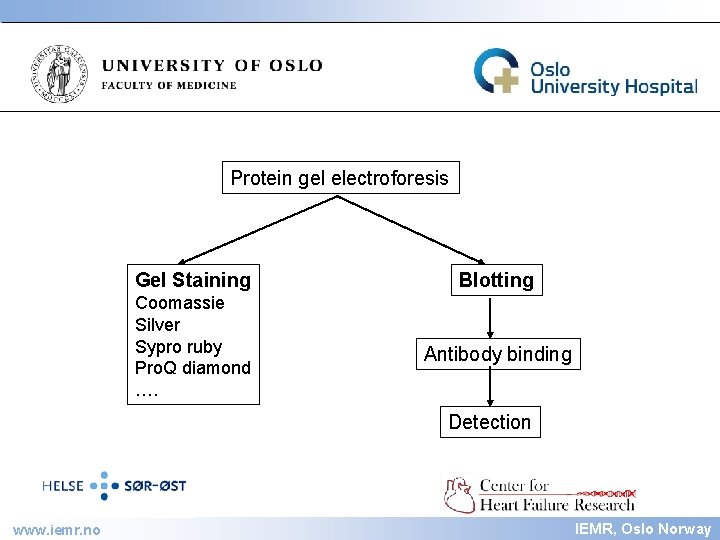
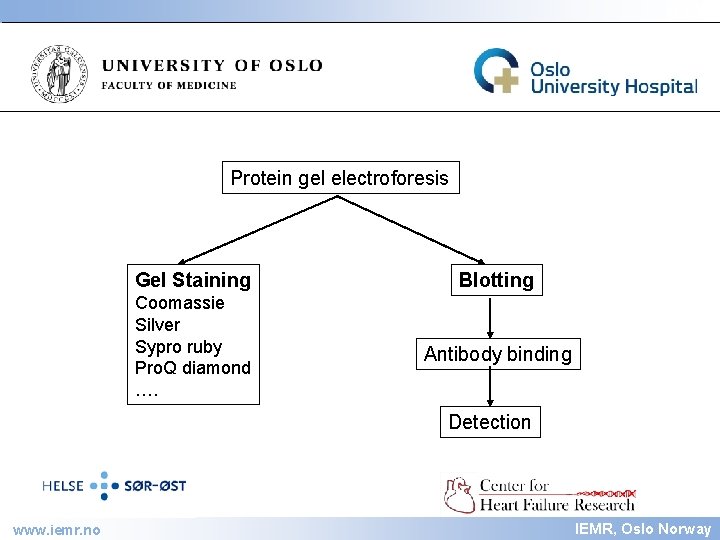
IEMR Protein gel electroforesis Gel Staining Blotting Coomassie Silver Sypro ruby Pro. Q diamond …. Antibody binding Detection www. iemr. no IEMR, Oslo Norway

IEMR Western blotting: - Method used for detection of a specific protein in a sample containing several proteins. - Based on antigen-antibody binding - Semi quantitativ www. iemr. no IEMR, Oslo Norway

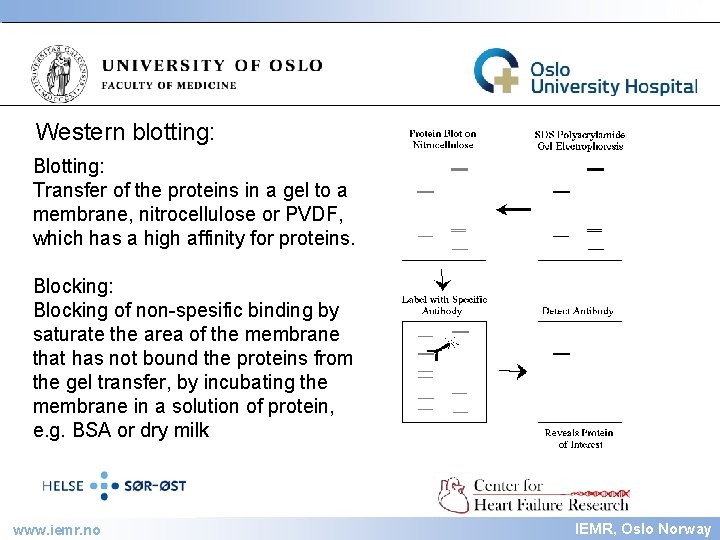
IEMR Western blotting: Blotting: Transfer of the proteins in a gel to a membrane, nitrocellulose or PVDF, which has a high affinity for proteins. Blocking: Blocking of non-spesific binding by saturate the area of the membrane that has not bound the proteins from the gel transfer, by incubating the membrane in a solution of protein, e. g. BSA or dry milk www. iemr. no IEMR, Oslo Norway

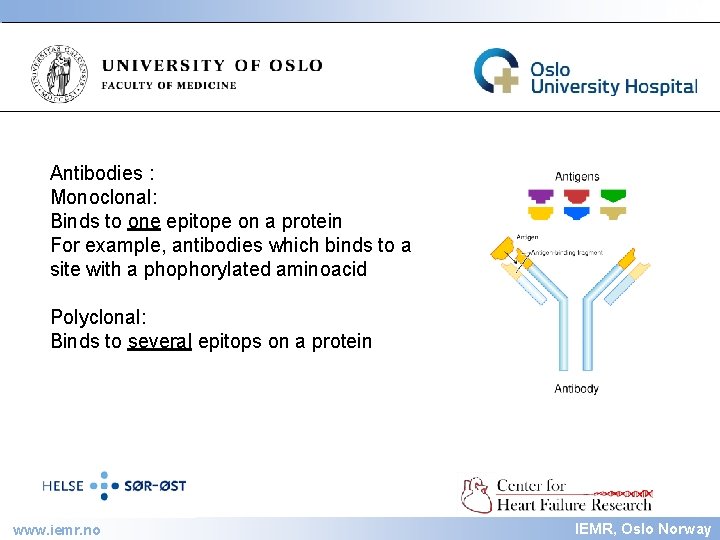
IEMR Antibodies : Monoclonal: Binds to one epitope on a protein For example, antibodies which binds to a site with a phophorylated aminoacid Polyclonal: Binds to several epitops on a protein www. iemr. no IEMR, Oslo Norway

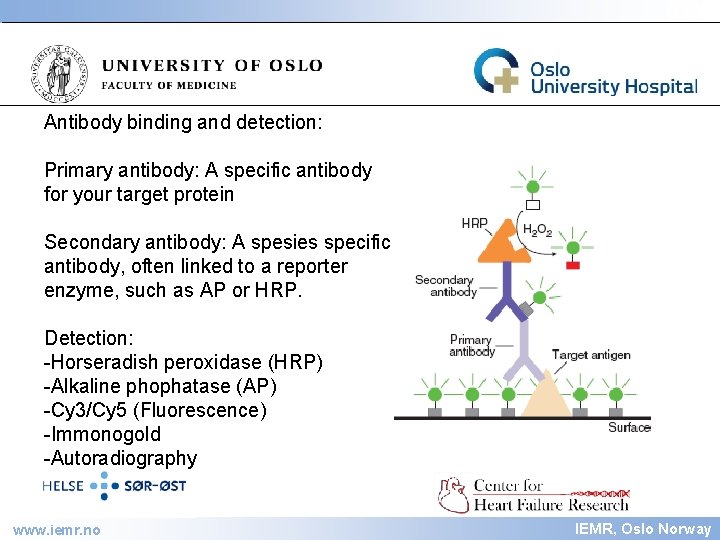
IEMR Antibody binding and detection: Primary antibody: A specific antibody for your target protein Secondary antibody: A spesies specific antibody, often linked to a reporter enzyme, such as AP or HRP. Detection: -Horseradish peroxidase (HRP) -Alkaline phophatase (AP) -Cy 3/Cy 5 (Fluorescence) -Immonogold -Autoradiography www. iemr. no IEMR, Oslo Norway

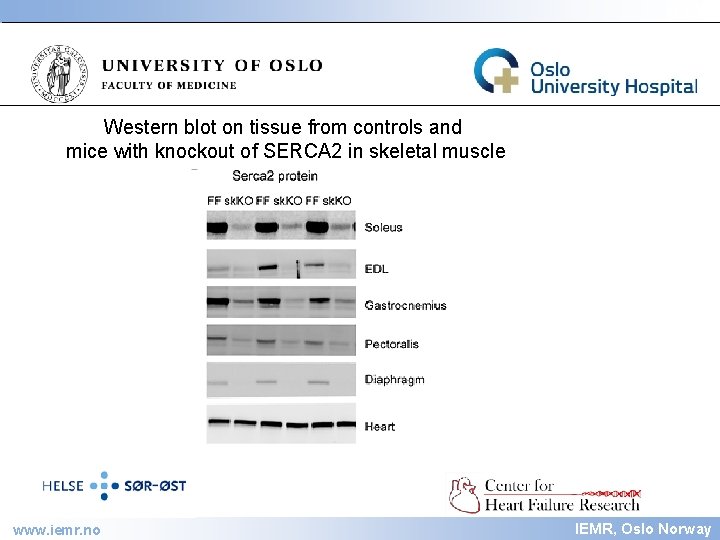
IEMR Western blot on tissue from controls and mice with knockout of SERCA 2 in skeletal muscle www. iemr. no IEMR, Oslo Norway

IEMR Protein gel electroforesis Gel Staining Blotting Coomassie Silver Sypro ruby Pro. Q diamond …. Antibody binding Detection www. iemr. no IEMR, Oslo Norway

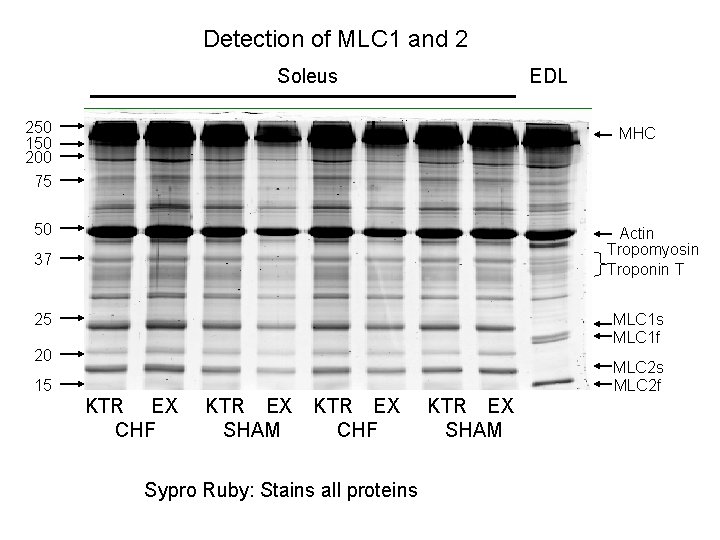
Detection of MLC 1 and 2 Soleus EDL 250 150 200 75 MHC 50 Actin Tropomyosin Troponin T 37 25 MLC 1 s MLC 1 f 20 MLC 2 s MLC 2 f 15 KTR EX CHF KTR EX SHAM KTR EX CHF Sypro Ruby: Stains all proteins KTR EX SHAM

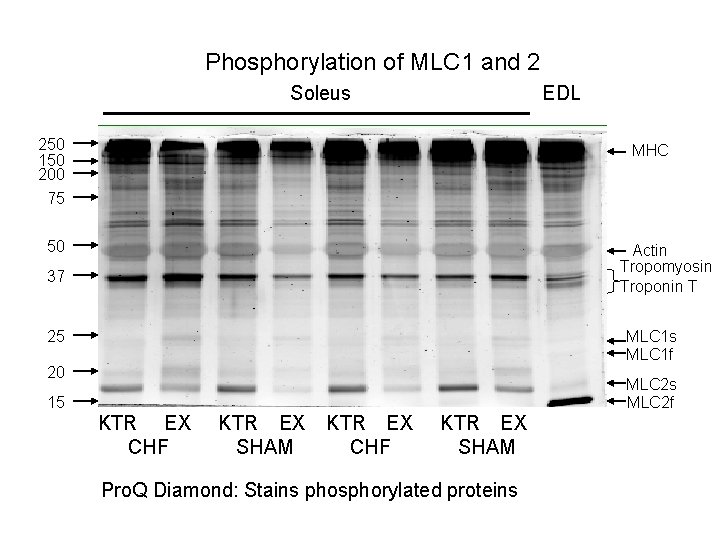
Phosphorylation of MLC 1 and 2 Soleus EDL 250 150 200 MHC 75 50 Actin Tropomyosin Troponin T 37 25 MLC 1 s MLC 1 f 20 MLC 2 s MLC 2 f 15 KTR EX CHF KTR EX SHAM Pro. Q Diamond: Stains phosphorylated proteins

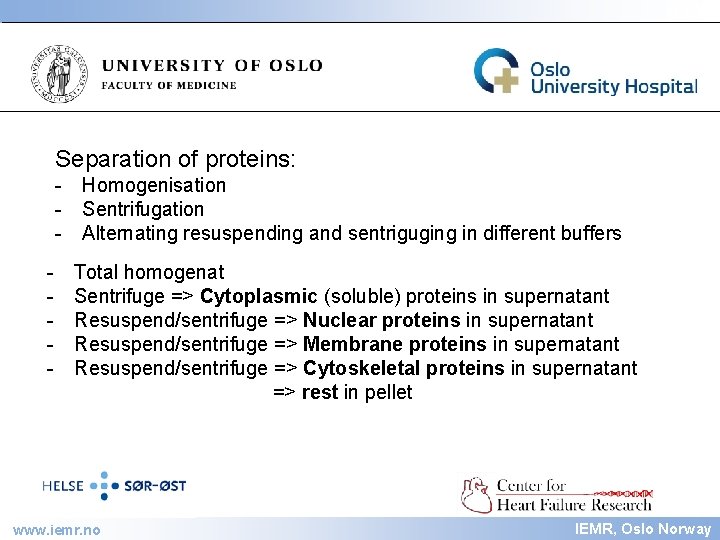
IEMR Separation of proteins: - Homogenisation - Sentrifugation - Alternating resuspending and sentriguging in different buffers - Total homogenat Sentrifuge => Cytoplasmic (soluble) proteins in supernatant Resuspend/sentrifuge => Nuclear proteins in supernatant Resuspend/sentrifuge => Membrane proteins in supernatant Resuspend/sentrifuge => Cytoskeletal proteins in supernatant => rest in pellet www. iemr. no IEMR, Oslo Norway

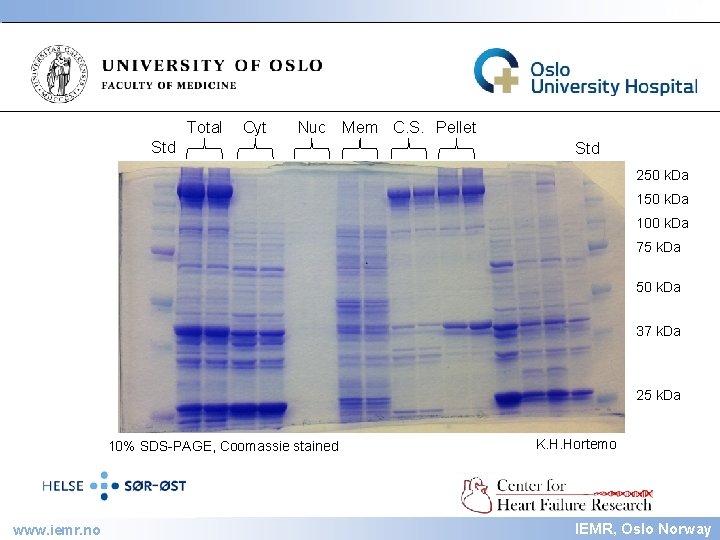
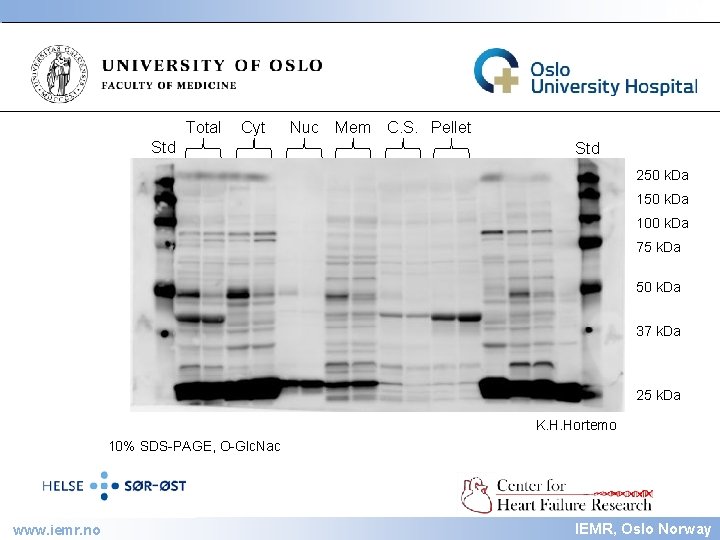
IEMR Total Cyt Nuc Std Mem C. S. Pellet Std 250 k. Da 100 k. Da 75 k. Da 50 k. Da 37 k. Da 25 k. Da 10% SDS-PAGE, Coomassie stained www. iemr. no K. H. Hortemo IEMR, Oslo Norway

IEMR Total Cyt Std Nuc Mem C. S. Pellet Std 250 k. Da 100 k. Da 75 k. Da 50 k. Da 37 k. Da 25 k. Da K. H. Hortemo 10% SDS-PAGE, O-Glc. Nac www. iemr. no IEMR, Oslo Norway

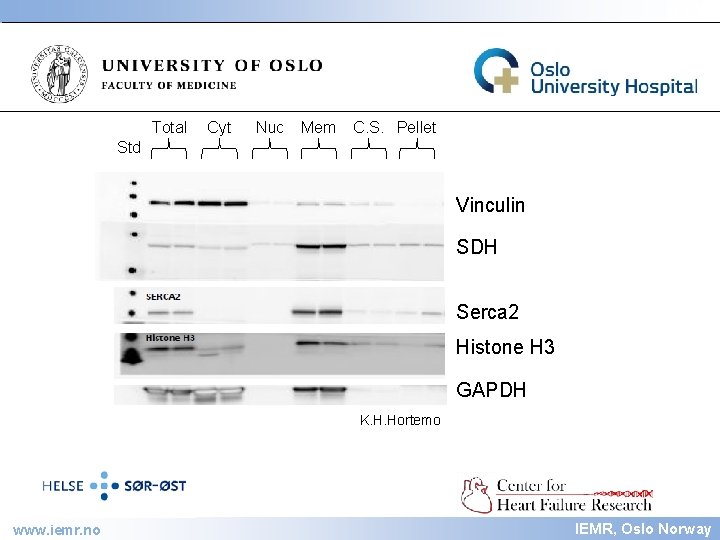
IEMR Total Cyt Nuc Mem C. S. Pellet Std Vinculin SDH Serca 2 Histone H 3 GAPDH K. H. Hortemo www. iemr. no IEMR, Oslo Norway

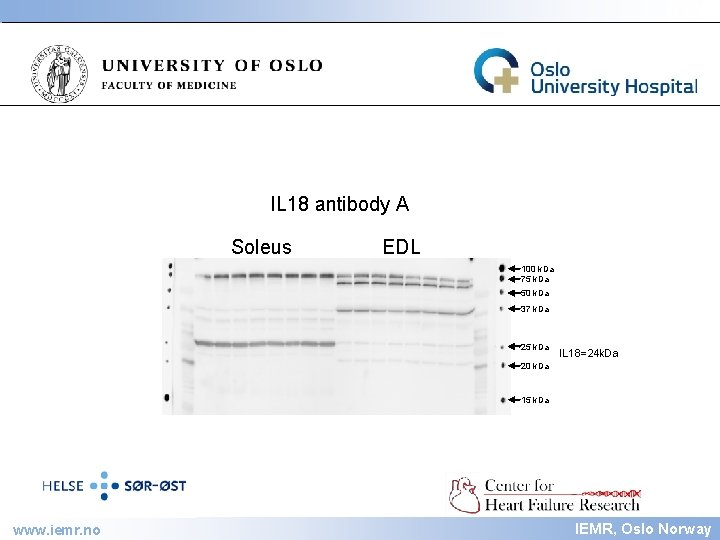
IEMR IL 18 antibody A Soleus EDL 100 k. Da 75 k. Da 50 k. Da 37 k. Da 25 k. Da IL 18=24 k. Da 20 k. Da 15 k. Da www. iemr. no IEMR, Oslo Norway

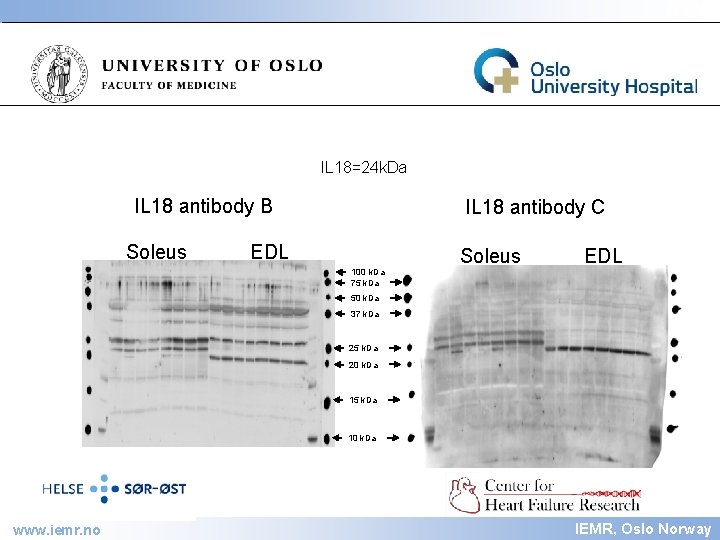
IEMR IL 18=24 k. Da IL 18 antibody B Soleus IL 18 antibody C EDL Soleus EDL 100 k. Da 75 k. Da 50 k. Da 37 k. Da 25 k. Da 20 k. Da 15 k. Da 10 k. Da www. iemr. no IEMR, Oslo Norway

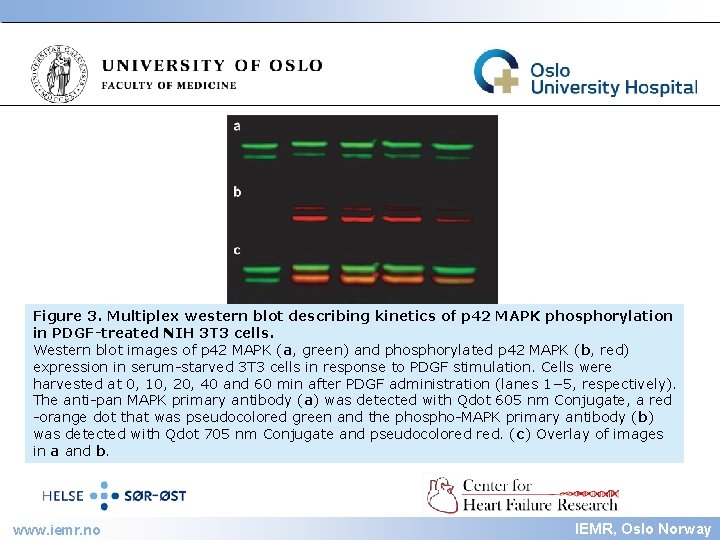
IEMR Figure 3. Multiplex western blot describing kinetics of p 42 MAPK phosphorylation in PDGF-treated NIH 3 T 3 cells. Western blot images of p 42 MAPK (a, green) and phosphorylated p 42 MAPK (b, red) expression in serum-starved 3 T 3 cells in response to PDGF stimulation. Cells were harvested at 0, 10, 20, 40 and 60 min after PDGF administration (lanes 1− 5, respectively). The anti-pan MAPK primary antibody (a) was detected with Qdot 605 nm Conjugate, a red -orange dot that was pseudocolored green and the phospho-MAPK primary antibody (b) was detected with Qdot 705 nm Conjugate and pseudocolored red. (c) Overlay of images in a and b. www. iemr. no IEMR, Oslo Norway