IEC TC 62 presentation to DICOM 2012 06











- Slides: 17

IEC TC 62 presentation to DICOM 2012 -06 -26 Norbert Bischof Secretary IEC TC 62 ELECTRICAL EQUIPMENT IN MEDICAL PRACTICE Secretary IEC SC 62 B DIAGNOSTIC IMAGING EQUIPMENT 1

International Medical Device Standards Content: International Standards Organisations ISO and IEC Technical Committee 62 IEC 62 standards 2

International Standardisation Organisations These organisations follow the WTOs* TBT** Code of Practice and accept one vote per country: ITU-T International Telecommunication Union ISO International Organisation for Standardisation IEC International Electrotechnical Commission However, there are many organisations producing “International Standards” , such as: IAEA, ASTM, BSI, . . . *WTO World Trade Organisation **TBT Technical Barriers to Trade 3

International Medical Device Standards Content: International Standards Organisations ISO and IEC Technical Committee 62 IEC 62 standards 4

ISO and IEC ISO International Organisation for Standardization, founded 1946 IEC International Electrotechnical Commission, founded 1906 § § § share a set of common directives § ONE member per nation standards fit seamlessly together are voluntary associations of MEMBER COUNTRIES establishing National Committees fully representing stakeholders in their countries: industry, government, consumers. . . , etc. 5

Medical Committees in ISO and IEC ISO/TC 76 ISO/TC 84 ISO/TC 106 ISO/TC 121 ISO/TC 150 ISO/TC 168 ISO/TC 170 ISO/TC 173 ISO/TC 184 ISO/TC 198 ISO/TC 210 ISO/TC 212 ISO/TC 215 Transfusion, infusion and injection equipment Administration of medicinal products and intravascular catheters Dentistry Anaesthetic and respiratory equipment Implants for surgery Prosthetics and orthotics Surgical instruments Assistive products for persons with disability Industrial automation systems and integration Biological evaluation of medical devices Sterilization of health care products Quality Management Systems for Medical Devices Clinical laboratory testing and in vitro diagnostic test systems Health informatics IEC/TC 62 Electrical equipment in medical practice 6

International Medical Device Standards Content: International Standards Organisations ISO and IEC Technical Committee 62 IEC 62 standards 7

TC 62 Electrical equipment in medical practice Established in 1968 Scope**: To prepare international standards and other publications concerning electrical equipment, electrical systems and software used in healthcare and their effects on patients, operators, other persons and the environment. NOTE: This scope includes items that are also within the scopes of other committees and will be addressed through cooperation. Attention will focus on safety and performance (e. g. radiation protection, data security, data integrity, data privacy and environmental aspects) and will contribute to regulatory frameworks. Healthcare includes medical practice as well as emergency medical services, homecare, and support of persons with disabilities in their daily lives (i. e. Ambient Assisted Living). ” ** as revised 2011 -09 -23 at the TC 62 meeting in Nuremberg and approved by the IEC Standards Management Board

Technical Committee 62 TC 62 Electrical equipment in medical practice CAG Chairman Advisory Group Chair Rudy Godinez / Sec DE Norbert Bischof SNAG Software and Networks Advisory Group SC 62 A Common aspects of electrical equipment used in medical practice Chair Justin Mc. Carthy Sec US Charles Sidebottom SC 62 B Diagnostic imaging equipment Chair Jim Malone Sec DE Norbert Bischof SC 62 C Radiotherapy, nuclear medicine and radiation dosimetry Chair Geoffrey S Ibbott Sec DE Claus-Peter Hoeppner SC 62 D Electromedical equipment Chair Robert Schaefer Sec US Mike Schmidt 9

IEC TC 62 Key Data* § § § § § Founded in 1968 116 active publications 18 publications last year 58 active projects 27 Participating Member Countries 17 Observing Member Countries 5 Committees (incl. the main Committee) 70 Working Groups 960 Experts from BE, BR, CA, CN, DK, FI, FR, DE, HU, IN, IE, IT, JP, NL, NO, PK, PT, RO, RU, ZA, ES, SE, CH, GB, US *per 2011 -11 -28

IEC TC 62 Meetings IEC TC 62 has met September 2011 in Nuremberg in conjunction with its subcommittees. The next meeting of IEC TC 62 and subcommittees will be held in Shanghai, China from April 8 to 19, 2013. 11

International Medical Device Standards Content: International Standards Organisations ISO and IEC Technical Committee 62 IEC 62 standards 12

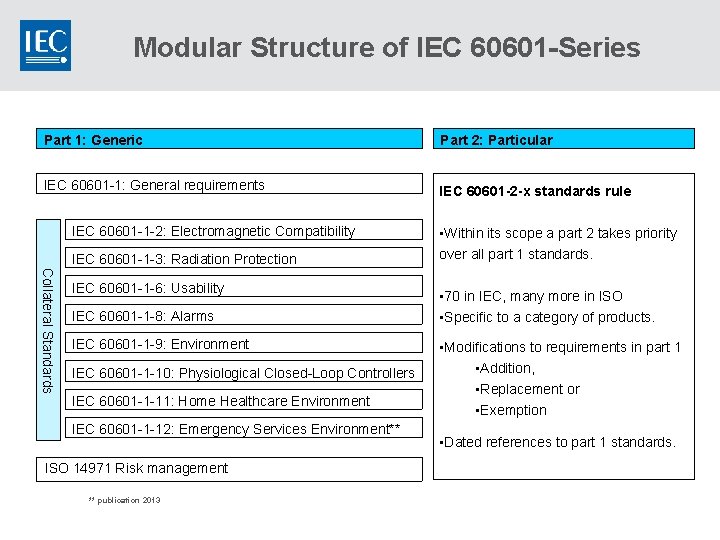
Modular Structure of IEC 60601 -Series Part 1: Generic Part 2: Particular IEC 60601 -1: General requirements IEC 60601 -2 -x standards rule IEC 60601 -1 -2: Electromagnetic Compatibility IEC 60601 -1 -3: Radiation Protection Collateral Standards IEC 60601 -1 -6: Usability IEC 60601 -1 -8: Alarms IEC 60601 -1 -9: Environment IEC 60601 -1 -10: Physiological Closed-Loop Controllers IEC 60601 -1 -11: Home Healthcare Environment IEC 60601 -1 -12: Emergency Services Environment** ISO 14971 Risk management ** publication 2013 • Within its scope a part 2 takes priority over all part 1 standards. • 70 in IEC, many more in ISO • Specific to a category of products. • Modifications to requirements in part 1 • Addition, • Replacement or • Exemption • Dated references to part 1 standards.

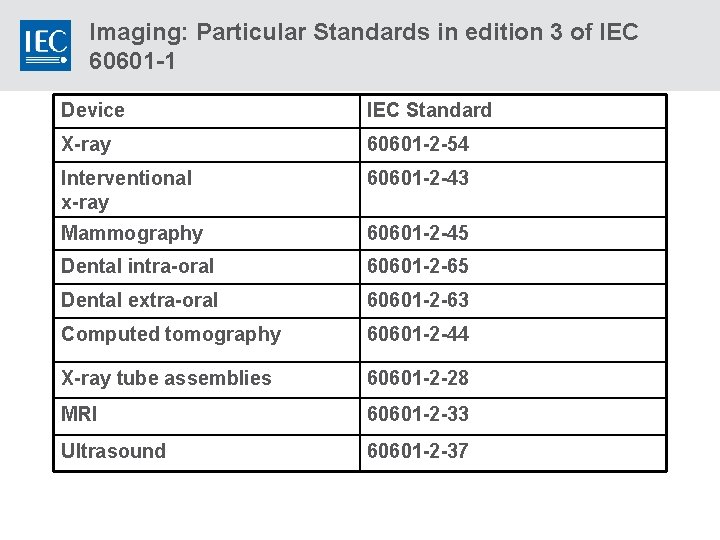
Imaging: Particular Standards in edition 3 of IEC 60601 -1 Device IEC Standard X-ray 60601 -2 -54 Interventional x-ray 60601 -2 -43 Mammography 60601 -2 -45 Dental intra-oral 60601 -2 -65 Dental extra-oral 60601 -2 -63 Computed tomography 60601 -2 -44 X-ray tube assemblies 60601 -2 -28 MRI 60601 -2 -33 Ultrasound 60601 -2 -37

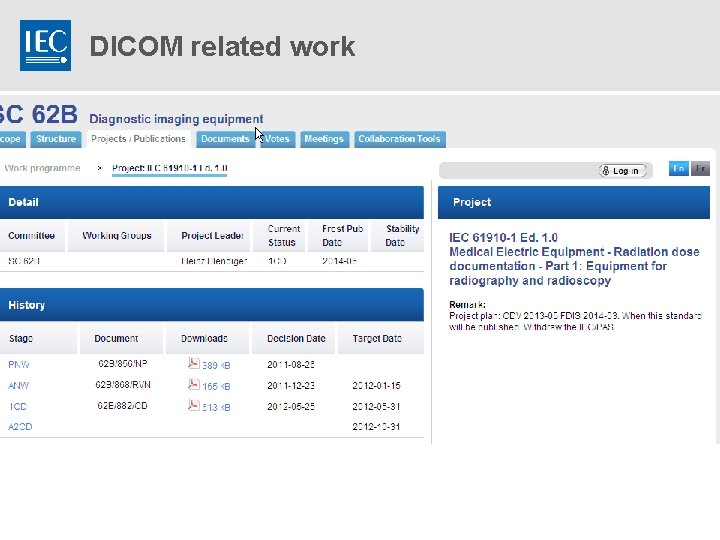
DICOM related work

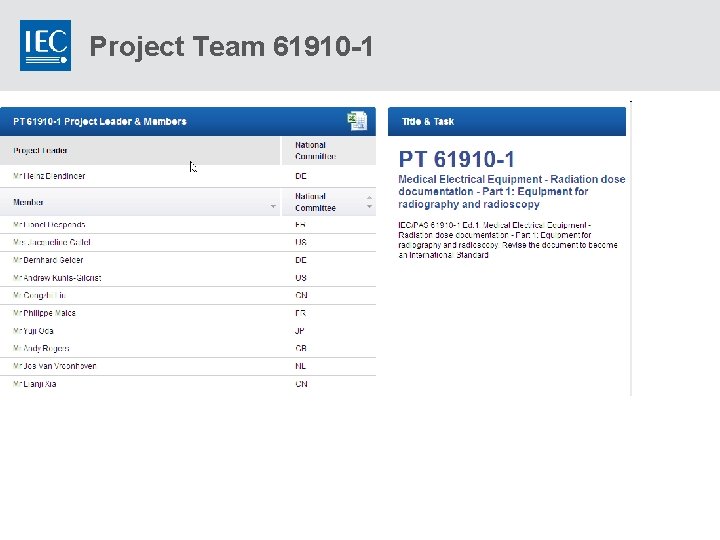
Project Team 61910 -1

Thank you Questions welcome Norbert Bischof bischof@cocir. org 17