IDO Inhibitors in Cancer Immunotherapy Geoffrey T Gibney

IDO Inhibitors in Cancer Immunotherapy Geoffrey T. Gibney, MD Co-leader, Melanoma Disease Group Georgetown Lombardi Comprehensive Cancer Center Medstar Georgetown University Hospital Washington, DC This activity is supported by an educational grant from Incyte Corporation

Disclosures Geoffrey T. Gibney, MD, has disclosed that he has received consulting fees from Genentech and Novartis and fees for non-CME/CE services from Merck.

About These Slides § Please feel free to use, update, and share some or all of these slides in your noncommercial presentations to colleagues or patients § When using our slides, please retain the source attribution: Slide credit: clinicaloptions. com § These slides may not be published, posted online, or used in commercial presentations without permission. Please contact permissions@clinicaloptions. com for details

Tumor Immune Phenotypes Inflamed Tumor Noninflamed Tumor IDO Reprinted by permission from Macmillan Publishers Ltd: Nature Immunology. Gajewski TF, et al. 2013; 14: 1014 -1022, © 2013. Slide credit: clinicaloptions. com

IDO 1: Indoleamine 2, 3 -Dioxygenase 1 Image reprinted from Clin Cancer Res, 2015; 21: 5427 -5433, Zhai L, et al, Molecular pathways: targeting IDO 1 and other tryptophan dioxygenases for cancer immunotherapy, Slide credit: clinicaloptions. com with permission from AACR.

IDO 1 Inhibitors in Clinical Development Class Phase Data Published or Presented Indoximod (NLG 2101, D-1 MT)*[1, 2] Selective IDO 1 inhibitor I-II Yes[3] Epacadostat (INCB 024360)*[4, 5] Selective IDO 1 inhibitor I-III Yes[6] GDC 0919 (NLG 919, RG 6078)[7] Selective IDO 1 inhibitor I Yes[8] IDO peptide vaccine* I Yes[10] Name IDO 5 peptide[9] *Stimulates immune response against IDO 1 -expressing cells. 1. NCT 01191216. 2. NCT 01792050. 3. Soliman HH, et al. Oncotarget. 2014; 5: 8136 -8146. 4. NCT 02178722. 5. NCT 02752074. 6. Gangadhar TC, et al. ESMO 2016. Abstract 1110 PD. 7. NCT 02048709. 8. Nayak A, et al. J Immunother Cancer. 2014; 2(suppl 3): P 250. 9. NCT 01219348. 10. Iversen TZ, et al. Basic Clin Pharmacol Toxicol. 2015; 116: 19 -24. Slide credit: clinicaloptions. com

Phase I Trial: IDO 1 Inhibitor Monotherapy With Indoximod § 3 + 3 dose-escalation study of indoximod in pts with advanced solid tumors, life expectancy > 4 mos (N = 48) – Dosed orally at 10 levels (200 mg, 300 mg, 400 mg, 600 mg, 800 mg daily; or 600 mg, 800 mg, 1200 mg, 1600 mg, 2000 mg BID) on 28 -day continuous cycle – Treatment continued until disease progression, toxicity, withdrawal – Safety assessed every 2 wks § Most frequent AEs were anemia, fatigue, anorexia, dyspnea, cough, and nausea, predominantly grade 1 or 2 § DLTs observed: hypophysitis (n = 3) § Doses up to 2000 mg BID well tolerated (MTD not reached) § No CR, no PR, 5 SD (10%) for at least 6 mos Soliman HH, et al. Oncotarget. 2016; 7: 22928 -22938. Slide credit: clinicaloptions. com

Phase I Trial: IDO 1 Inhibitor Monotherapy With Epacadostat § 3 + 3 dose-escalation study in pts with advanced malignancies (N = 52) – Dosed orally in 28 -day cycles (50 mg daily; 50 mg, 100 mg, 300 mg, 400 mg, 500 mg, 600 mg, 700 mg BID) – Treatment continued until disease progression, toxicity – PK/PD assessed on Days 1, 15 § Most frequent grade 3/4 AEs were fatigue (11. 5%), abdominal pain (9. 6%), and hypokalemia (9. 6%) § 2 DLTs observed: radiation pneumonitis (n = 1) and fatigue (n = 1) § Doses up to 700 mg BID well tolerated § No CR, no PR, 8 SD (15. 4%) at ≥ 16 wks Beatty GL, et al. ASCO 2013. Abstract 3205. Slide credit: clinicaloptions. com

Phase I Vaccination Trial: IDO 1 Inhibitor Monotherapy With IDO 5 Peptide Vaccine § Phase I study of an IDO 5 peptide vaccine given monthly in 15 pts with HLA-A 2–positive non-small-cell lung cancer § Median OS = 25. 9 months; differences in baseline characteristics may have influenced results § One pt with IDO 5 peptide vaccine had PR and 6 others achieved SD for more than 8. 5 months § Treatment well tolerated; no reported grade 3/4 events Iversen TZ, et al. Clin Cancer Res. 2014; 20: 221 -232.

IDO 1 Inhibitor Combination Strategies Chemotherapy IDO 1 inhibitor Plus Vaccine/ immune stimulus Immune checkpoint inhibitor Slide credit: clinicaloptions. com

Upregulation of IDO 1 Can Occur Concurrently With Other Immunosuppressive Mechanisms PD-L 1 Fox. P 3 CD 8 Pt 2 Pt 1 IDO Image from Spranger S, et al. Up-regulation of PD-L 1, IDO, and T(regs) in the melanoma tumor microenvironment is driven by CD 8(+) T cells. Sci Transl Med. 2013; 5: 200 ra 116. Reprinted with permission from AAAS. Readers may view, browse, and/or download material for temporary copying purposes only, provided these uses are for noncommercial personal purposes. Except as provided by law, this material may not be further reproduced, distributed, transmitted, modified, adapted, performed, displayed, published, or sold in whole or in part, without prior written permission from the publisher. Slide credit: clinicaloptions. com

Preclinical Data With Combination IDO 1 and Immune Checkpoint Inhibition No treatment Anti–PD-L 1 IDO inhibitor Anti–CTLA-4 + IDO inhibitor 500 400 300 200 100 0 No treatment Anti–CTLA-4 7 14 21 26 Days After Tumor Inoculation Spranger S, et al. J Immunother Cancer. 2014; 2: 3. 0 7 IDO inhibitor Anti–PD-L 1 + IDO inhibitor 14 21 24 Days After Tumor Inoculation Slide credit: clinicaloptions. com
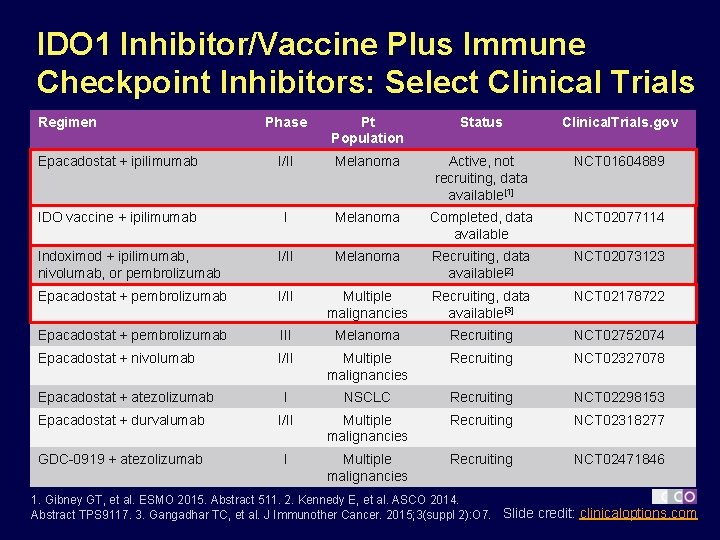
IDO 1 Inhibitor/Vaccine Plus Immune Checkpoint Inhibitors: Select Clinical Trials Regimen Phase Pt Population Status Clinical. Trials. gov Epacadostat + ipilimumab I/II Melanoma Active, not recruiting, data available[1] NCT 01604889 IDO vaccine + ipilimumab I Melanoma Completed, data available NCT 02077114 Indoximod + ipilimumab, nivolumab, or pembrolizumab I/II Melanoma Recruiting, data available[2] NCT 02073123 Epacadostat + pembrolizumab I/II Multiple malignancies Recruiting, data available[3] NCT 02178722 Epacadostat + pembrolizumab III Melanoma Recruiting NCT 02752074 Epacadostat + nivolumab I/II Multiple malignancies Recruiting NCT 02327078 I NSCLC Recruiting NCT 02298153 Epacadostat + durvalumab I/II Multiple malignancies Recruiting NCT 02318277 GDC-0919 + atezolizumab I Multiple malignancies Recruiting NCT 02471846 Epacadostat + atezolizumab 1. Gibney GT, et al. ESMO 2015. Abstract 511. 2. Kennedy E, et al. ASCO 2014. Abstract TPS 9117. 3. Gangadhar TC, et al. J Immunother Cancer. 2015; 3(suppl 2): O 7. Slide credit: clinicaloptions. com

Phase I/II Study of Epacadostat Plus Ipilimumab in Melanoma: Design § Pts (N = 42) with unresectable or metastatic melanoma, life expectancy ≥ 3 mos, ECOG PS 0 or 1 § Initial phase I dose-escalation study of 300 -mg BID epacadostat plus 4 doses of ipilimumab (3 mg/kg IV Q 3 W) – Clinically significant ALT elevations in 5/7 pts; all recovered § Design amended to lower dose of epacadostat (25 mg BID, 50 mg BID continuous; 50 mg BID intermittent; 75 mg total daily) plus ipilimumab (3 mg/kg IV Q 3 W x 4) Gibney GT, et al. ESMO 2015. Abstract 511. Slide credit: clinicaloptions. com

Epacadostat Plus Ipilimumab in Melanoma: Baseline Characteristics Pt Characteristic 25 mg BID (n = 8) 50 mg BID Continuous (n = 18) 50 mg BID Intermittent (n = 9) 75 mg Total Daily Dose (n = 7) Age, median yrs (range) 67 (34 -81) 57 (25 -78) 69 (49 -77) 62 (35 -81) 75. 0 50. 0 66. 7 42. 9 ECOG PS 0/1, % 62. 5/37. 5 88. 9/11. 1 66. 7/33. 3 85. 7/14. 3 Mutated BRAF, % 25. 0 44. 4 22. 2 14. 3 Prior radiotherapy, % 50. 0 44. 4 22. 2 42. 9 M classification, % § 0 § 1 a § 1 b § 1 c 12. 5 25. 0 50. 0 0 27. 8 22. 2 44. 4 22. 2 0 11. 1 66. 7 0 42. 9 14. 3 42. 9 Male, % Gibney GT, et al. ESMO 2015. Abstract 511. Slide credit: clinicaloptions. com

Epacadostat Plus Ipilimumab in Melanoma: Prior Regimens 25 mg BID (n = 8) 50 mg BID Continuous (n = 18) 50 mg BID Intermittent (n = 9) 75 mg Total Daily Dose (n = 7) Prior regimens, % § None § 1 -2 § ≥ 3 100 0 0 55. 6 27. 8 16. 7 66. 7 33. 3 0 71. 4 28. 6 0 Regimen types, % § Immunotherapy • Checkpoint inhibitor • IL-2 • Other § BRAF inhibitor § Chemotherapy § Other 0 0 0 26. 7 11. 1 22. 2 16. 7 11. 1 22. 2 11. 1 0 0 0 14. 3 28. 6 Regimen Gibney GT, et al. ESMO 2015. Abstract 511. Slide credit: clinicaloptions. com

Epacadostat Plus Ipilimumab in Melanoma: Safety 25 mg (n = 8) 50 mg Continuous (n = 18) 50 mg Intermittent (n = 9) 75 mg (n = 7) Total (N = 42)* Rash 62. 5 55. 6 11. 1 85. 7 52. 4 Pruritus 25. 0 33. 3 55. 6 42. 9 38. 1 Diarrhea 37. 5 33. 3 22. 2 42. 9 33. 3 ALT increased 25. 0 16. 7 22. 2 28. 6 21. 4 AST increased 25. 0 16. 7 11. 1 14. 3 16. 7 Hypothyroidism 25. 0 16. 7 0 0 11. 9 Colitis 25. 0 5. 6 11. 1 0 9. 5 AE (all grades), % *Other AEs (total): adrenal insufficiency (7. 1%), vitiligo (7. 1%), acute renal failure (4. 8%), hypophysitis (4. 8%), hypopituitarism (2. 4%), idiopathic thrombocytopenic purpura (2. 4%), pneumonitis (2. 4%). Gibney GT, et al. ESMO 2015. Abstract 511. Slide credit: clinicaloptions. com
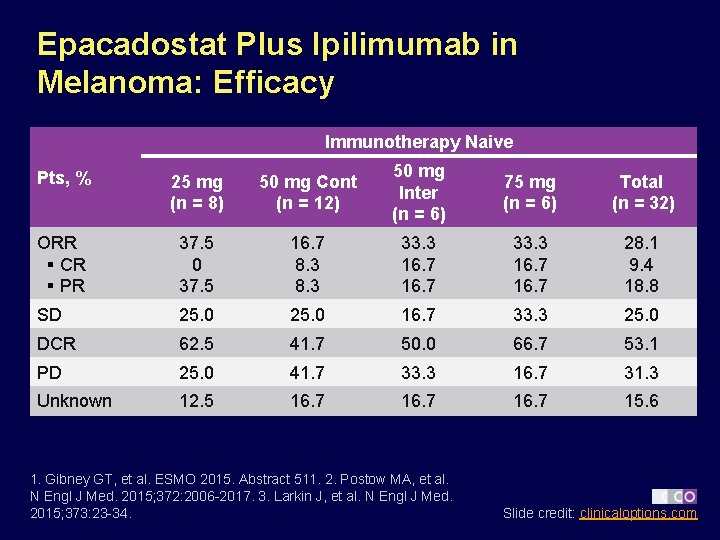
Epacadostat Plus Ipilimumab in Melanoma: Efficacy Immunotherapy Naive Pts, % 25 mg (n = 8) 50 mg Cont (n = 12) 50 mg Inter (n = 6) 75 mg (n = 6) Total (n = 32) ORR § CR § PR 37. 5 0 37. 5 16. 7 8. 3 33. 3 16. 7 28. 1 9. 4 18. 8 SD 25. 0 16. 7 33. 3 25. 0 DCR 62. 5 41. 7 50. 0 66. 7 53. 1 PD 25. 0 41. 7 33. 3 16. 7 31. 3 Unknown 12. 5 16. 7 15. 6 1. Gibney GT, et al. ESMO 2015. Abstract 511. 2. Postow MA, et al. N Engl J Med. 2015; 372: 2006 -2017. 3. Larkin J, et al. N Engl J Med. 2015; 373: 23 -34. Slide credit: clinicaloptions. com

Phase I Study of IDO Vaccine Plus Ipilimumab in Melanoma § Pts with stage III/IV melanoma, ECOG PS ≤ 2 (N = 10); pts (n = 9) systemically therapy naive (1 pt IFN) § Dosed with ipilimumab (3 mg/kg IV Q 3 W x 4) plus vaccinated with IDO 21 -mer peptide in Montanide (SC, weekly x 4, then biweekly x 3) § Endpoints: safety, vaccine response, clinical response – Blood drawn 5 x (baseline, Wks 3, 7, 12, 20 -24, 28 -36) – PET/CT performed 4 x (baseline, Wks 12, 20 -24, 28 -36) Bjoern J, et al. Cytotherapy. 2016; 18: 1043 -1055. Slide credit: clinicaloptions. com

IDO Vaccine Plus Ipilimumab in Melanoma: Outcomes § No grade 3/4 AEs; 1 grade 5 colitis event observed after pt declined hospital readmission/infliximab in the corticosteroid-refractory setting § Local vaccine injection-site reactions, rash, pruritus, and diarrhea were most common (grade 1/2) § 5 SD; 1 pt with unconfirmed PR, then progression in nontarget lesions Bjoern J, et al. Cytotherapy. 2016; 18: 1043 -1055. Slide credit: clinicaloptions. com

Phase Ib/II: Indoximod Plus Ipilimumab, Nivolumab, or Pembrolizumab § Phase Ib study in pts with melanoma (N = 9): (3 pts at 600 mg BID, 6 pts at 1200 mg BID) plus ipilimumab (3 mg/kg Q 3 W x 4) § Outcomes: – No DLTs; indoximod plus ipilimumab well tolerated; most common AEs fatigue, pruritus, diarrhea, rash, abdominal pain, headache – 1 CR – Phase II study in pts with melanoma (N = 96 planned): indoximod (1200 mg BID) plus immune checkpoint inhibitor (4 cycles of ipilimumab, repeat cycles of nivolumab, repeat cycles of pembrolizumab) § On disease progression, provider can switch to another checkpoint inhibitor while continuing indoximod Zakharia Y, et al. ASCO 2016. Abstract 3075. Slide credit: clinicaloptions. com
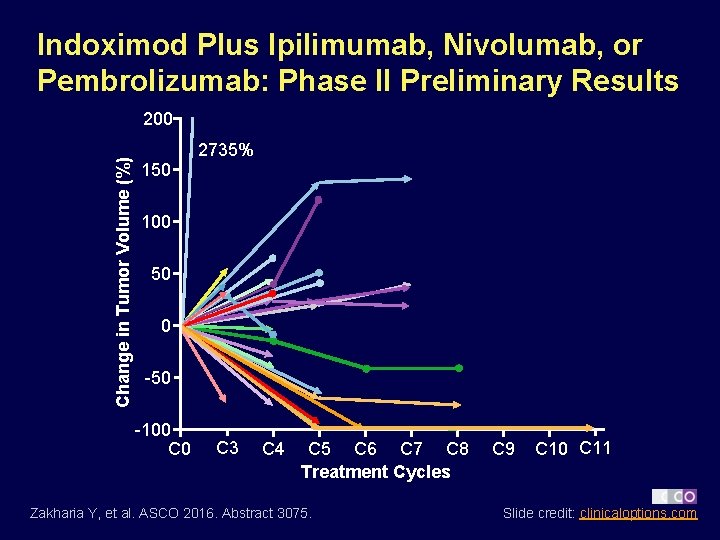
Indoximod Plus Ipilimumab, Nivolumab, or Pembrolizumab: Phase II Preliminary Results Change in Tumor Volume (%) 200 2735% 150 100 50 0 -50 -100 C 3 C 4 C 5 C 6 C 7 C 8 Treatment Cycles Zakharia Y, et al. ASCO 2016. Abstract 3075. C 9 C 10 C 11 Slide credit: clinicaloptions. com

Phase Ib/II Epacadostat Plus Pembrolizumab: Study Design § Dose-escalation/dose-expansion study in pts with advanced cancers, life expectancy > 12 wks, ECOG PS 0/1, checkpoint inhibitor naive (N = 54) – Dose-escalation phase of epacadostat (25 mg, 50 mg, 100 mg, 300 mg BID) plus pembrolizumab (2 mg/kg Q 3 W) – Dose-expansion phase of epacadostat (50 mg, 100 mg, 300 mg BID) plus pembrolizumab (200 mg Q 3 W) § Responses assessed Q 9 W per RECIST 1. 1 Gangadhar TC, et al. SITC 2015. Abstract 142. Slide credit: clinicaloptions. com

Phase Ib/II Study of Epacadostat Plus Pembrolizumab: Baseline Characteristics Tumor type, % § Melanoma § RCC § NSCLC § TCC § EA § TNBC § SCCHN Total (N = 56) 25 mg (n = 4) 50 mg (n = 19) 100 mg (n = 18) 300 mg (n = 15) 36 20 18 9 9 5 4 50 0 25 25 0 0 0 68 21 0 0 5 22 6 39 17 11 0 6) 7 40 13 7 13 20 0 47 (30 -63) 60 (37 -81) 61. 5 (39 -88) 59 (30 -84) Median age, yrs 59 (30 -88) (range) Male, % 55 25 53 66 53 ECOG PS, % § 0 § 1 57 43 100 0 74 26 56 44 27 73 Gangadhar TC, et al. SITC 2015. Abstract 142. Slide credit: clinicaloptions. com
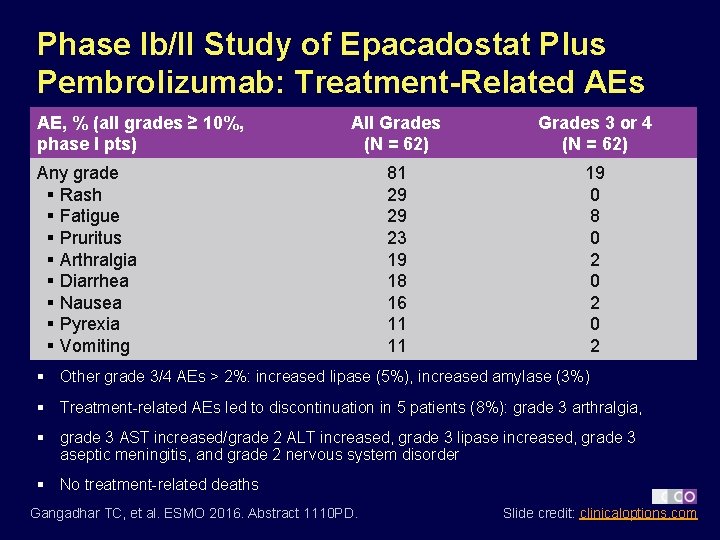
Phase Ib/II Study of Epacadostat Plus Pembrolizumab: Treatment-Related AEs AE, % (all grades ≥ 10%, phase I pts) All Grades (N = 62) Grades 3 or 4 (N = 62) 81 29 29 23 19 18 16 11 11 19 0 8 0 2 0 2 Any grade § Rash § Fatigue § Pruritus § Arthralgia § Diarrhea § Nausea § Pyrexia § Vomiting § Other grade 3/4 AEs > 2%: increased lipase (5%), increased amylase (3%) § Treatment-related AEs led to discontinuation in 5 patients (8%): grade 3 arthralgia, § grade 3 AST increased/grade 2 ALT increased, grade 3 lipase increased, grade 3 aseptic meningitis, and grade 2 nervous system disorder § No treatment-related deaths Gangadhar TC, et al. ESMO 2016. Abstract 1110 PD. Slide credit: clinicaloptions. com

Phase Ib/II Study of Epacadostat Plus Pembrolizumab: Efficacy Melanoma (n = 19) RCC (n = 11) NSCLC (n = 12) TCC (n = 5) EA (n = 7) TNBC (n = 3) SCCHN (n = 2) ORR, % § CR § PR 58 26 32 18 0 18 42 0 42 60 0 60 28 14 14 0 0 0 50 SD, % 16 45 17 0 0 67 50 DCR, % 74 63 59 60 28 67 100 Gangadhar TC, et al. ESMO 2016. Abstract 1110 PD. Slide credit: clinicaloptions. com
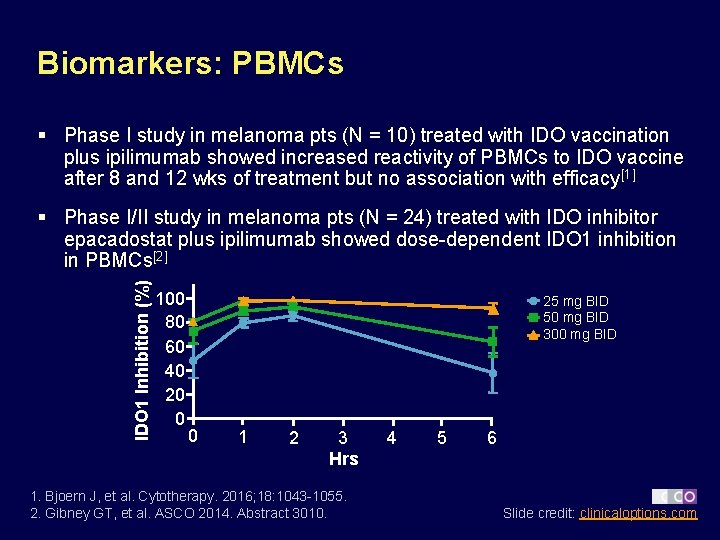
Biomarkers: PBMCs § Phase I study in melanoma pts (N = 10) treated with IDO vaccination plus ipilimumab showed increased reactivity of PBMCs to IDO vaccine after 8 and 12 wks of treatment but no association with efficacy[1] IDO 1 Inhibition (%) § Phase I/II study in melanoma pts (N = 24) treated with IDO inhibitor epacadostat plus ipilimumab showed dose-dependent IDO 1 inhibition in PBMCs[2] 100 80 60 40 20 0 25 mg BID 50 mg BID 300 mg BID 0 1 2 3 Hrs 1. Bjoern J, et al. Cytotherapy. 2016; 18: 1043 -1055. 2. Gibney GT, et al. ASCO 2014. Abstract 3010. 4 5 6 Slide credit: clinicaloptions. com
![Other Biomarkers NSCLC Cohort[1] Melanoma Cohort[1] Best Change From Baseline (%) 100 PD-L 1 Other Biomarkers NSCLC Cohort[1] Melanoma Cohort[1] Best Change From Baseline (%) 100 PD-L 1](http://slidetodoc.com/presentation_image_h/02901aca4841c0561f06155609a6aac6/image-28.jpg)
Other Biomarkers NSCLC Cohort[1] Melanoma Cohort[1] Best Change From Baseline (%) 100 PD-L 1 positive PD-L 1 negative PD-L 1 unknown 100 50 50 0 0 -50 -100 Pts PD-L 1 positive PD-L 1 negative Pts § Phase Ib/II study of epacadostat plus pembrolizumab found unclear association between PD-L 1 tumor status and tumor burden reduction[1] § IHC for IDO 1 has been standardized in clinical samples, but data not available on association with clinical activity[2] 1. Gangadhar TC, et al. ESMO 2016. Abstract 1110 PD. 2. Hiscox A, et al. ASCO 2014. Abstract 3043. PD-L 1 unknown

Conclusions § IDO 1 selective inhibitors and IDO vaccine are well tolerated but demonstrate modest clinical activity as monotherapy in pts with advanced malignancies[1, 2] § Preclinical data indicate synergistic potential for combined IDO 1 inhibition plus immunotherapies (and chemotherapy)[3] § No early signs of increased serious toxicity with IDO 1 inhibition plus immune checkpoint therapy[4] § IDO 1 inhibition plus anti–PD-1 therapy shows promising early clinical activity, especially in advanced melanoma patients; phase III study ongoing[5] § Further biomarker development is needed for combination IDO 1 inhibition therapeutic strategies 1. Beatty GL, et al. ASCO 2013. Abstract 3205. 2. Bjoern J, et al. Cytotherapy. 2016; 18: 1043 -1055. 3. Spranger S, et al. J Immunother Cancer. 2014; 2: 3. 4. Gibney GT, et al. ESMO 2015. Abstract 511. 5. Clinical. Trials. gov. NCT 02752074. Slide credit: clinicaloptions. com

Go Online for More CCO Coverage of Cancer Immunotherapy! Capsule Summaries of all the key data Additional CME-certified slidesets, modules, and videos on Immunotherapy with expert faculty commentary on key studies clinicaloptions. com/oncology
- Slides: 30