IDMP and SPOR what are they and what








- Slides: 24

IDMP and SPOR – what are they and what is going on? Dr Jeffrey Martin, Enterprise Architect, IT Business Support Group, Information Technology Department, Swedish Medical Products Agency

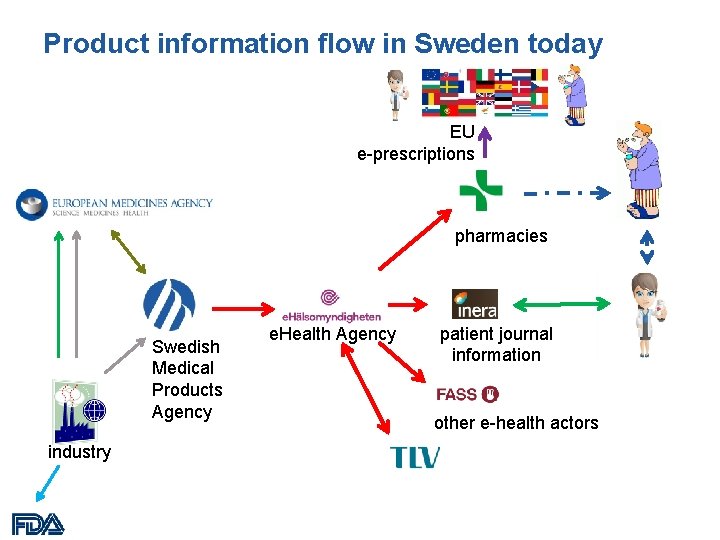
Product information flow in Sweden today EU e-prescriptions pharmacies Swedish Medical Products Agency industry e. Health Agency patient journal information other e-health actors

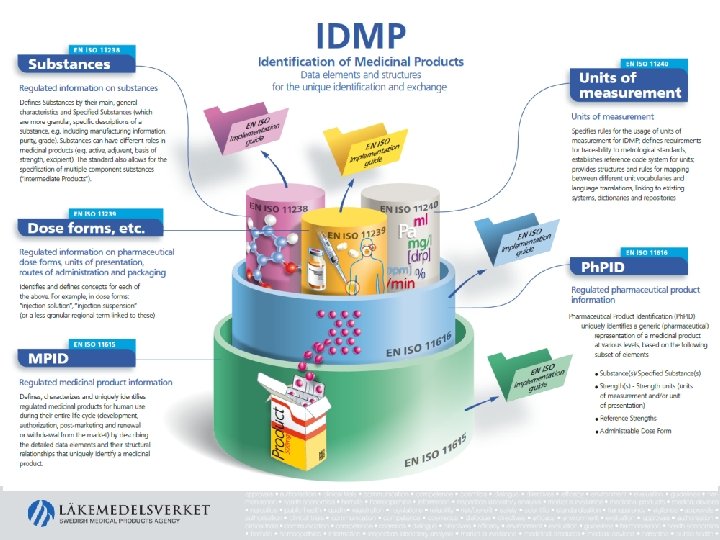
A new standard for information: IDMP – Identification of Medicinal Products • 5 ISO/CEN-standards that define how (human) medicinal product information shall be structured • Standards approved 2012/2017/2018, global implementation guides 2017/2018 • Mandatory in the EU for adverse reaction reporting eventually – Commission Implementing Regulation 520/2012, articles 25 and 26 (pharmacovigilance regulation)


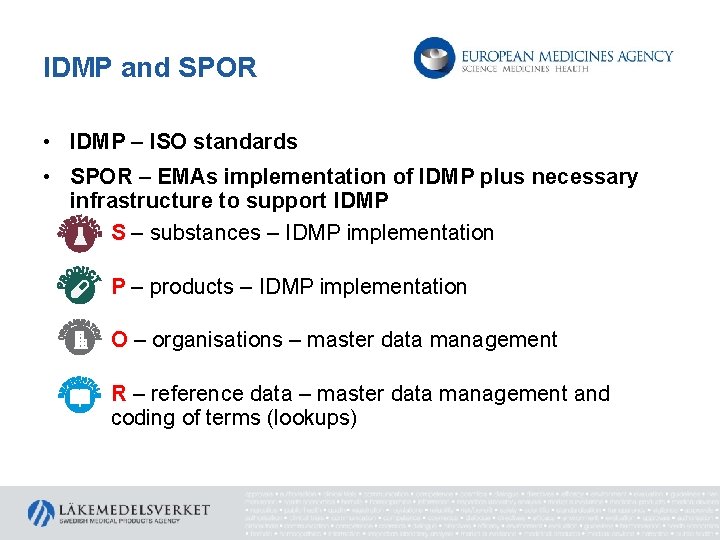
IDMP and SPOR • IDMP – ISO standards • SPOR – EMAs implementation of IDMP plus necessary infrastructure to support IDMP S – substances – IDMP implementation P – products – IDMP implementation O – organisations – master data management R – reference data – master data management and coding of terms (lookups)

Common EU medicinal product database: ”Article 57” data • Regulation 726/2004 (upd 1235/2010) specifies that all marketing authorisation holders must submit product information for all their human products in to EMA Eudra. Vigilance for vigilance purposes – – From July 2012 Continuously updated by Marketing Authorisation Holders (MAHs) > 90% of all products Many thousands of substances without quality control • EMA has done some cleaning but quality still not optimal – ca 500 000 products from ca 4 600 MAHs • Technical validation • Business validation via manual cross-check with Sm. PC – No national European Agency involvement – Extremely laborious • Start of a common EU database with potential use for prescriptions, regulatory activities, vigilance etc. For the purposes of the database, the Agency shall set up and maintain a list of all medicinal products for human use authorised in the Union.

”Article 57” data – XEVMPD ISO IDMP ”Product Master System” • Art 57 data is today in the format ”XEVMPD” – e. Xtended Eudra. Vigilance Medical Products Dictionary – Eudra. Vigilance is EMAs system into which all companies and agencies must report adverse reactions – XEVMPD format is based on a preliminary version of ISO IDMP which was not ready when the regulation went into effect (2011 -2012) – EMA will convert XEVMPD-data to ISO IDMP but companies must follow and submit in IDMP after that • Ca 2022

Conversion and Mapping of Products • In Sweden, > 15 000 approved products to deal with (including ca 1 000 veterinary products) – Some very old products, e. g. Aspirin® approved 1935 – Homeopathics • EMA has > 500 000 products in Art 57 data to convert from ca 4 600 data providers! • Only a subset of all possible IDMP product information will be in PMS • Different European Agencies have different amounts of structured information – Ingredients, packages – Structured clinical information almost universally lacking

Information Quality • Data from European Agencies go directly to prescription systems – Need for very high quality data – virtually 100% correct • EMA has the ambition that Art 57 data (from PMS) will be the basis for EU-wide medicines information – Cross-border patient services – Data must be up-to-date – Accurate • Important to increase the quality and timeliness of the information at EMA

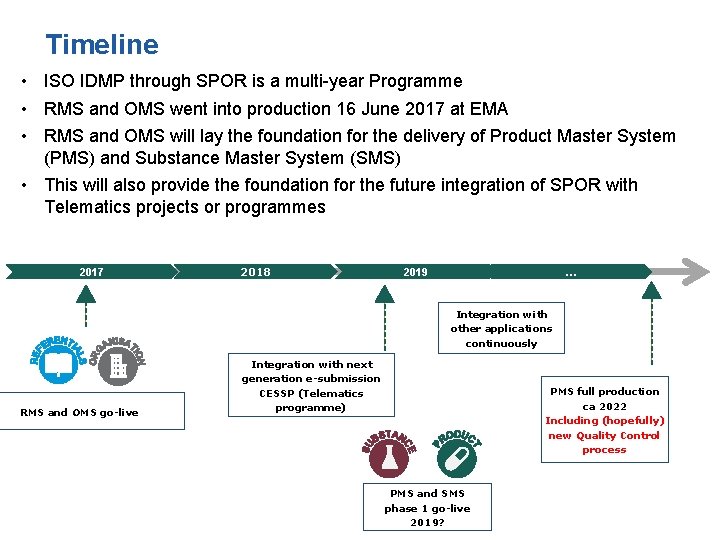
High level programme timeline Timeline • ISO IDMP through SPOR is a multi-year Programme • RMS and OMS went into production 16 June 2017 at EMA • RMS and OMS will lay the foundation for the delivery of Product Master System (PMS) and Substance Master System (SMS) • This will also provide the foundation for the future integration of SPOR with Telematics projects or programmes 2017 2018 2019 … Integration with other applications continuously RMS and OMS go-live Integration with next generation e-submission CESSP (Telematics programme) PMS full production ca 2022 Including (hopefully) new Quality Control process PMS and SMS phase 1 go-live 2019?

When will we be ready? • Substances – – – European codes for substances Project is starting up under NL leadership A Substance Validation Group will start work on these 2019 Not just codes but quality assurance Probably take a couple of years to get most substances done • Dosage form codes – Most Agencies have done the mapping to EDQM/RMS codes – Lots of unresolved issues about special national codes

SPOR Change Liaisons • Almost every country/National Agency has a SPOR Change Liaison • Contact them via your National Agency • List of contacts will be sent out soon

Short description of IDMP

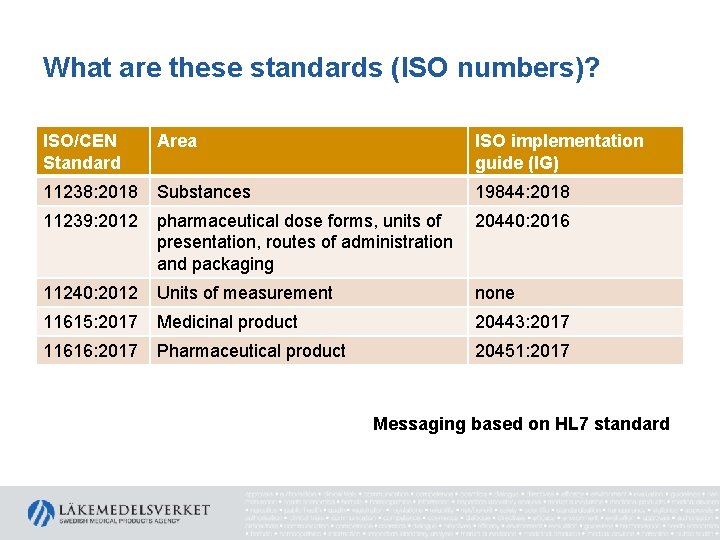
What are these standards (ISO numbers)? ISO/CEN Standard Area ISO implementation guide (IG) 11238: 2018 Substances 19844: 2018 11239: 2012 pharmaceutical dose forms, units of presentation, routes of administration and packaging 20440: 2016 11240: 2012 Units of measurement none 11615: 2017 Medicinal product 20443: 2017 11616: 2017 Pharmaceutical product 20451: 2017 Messaging based on HL 7 standard

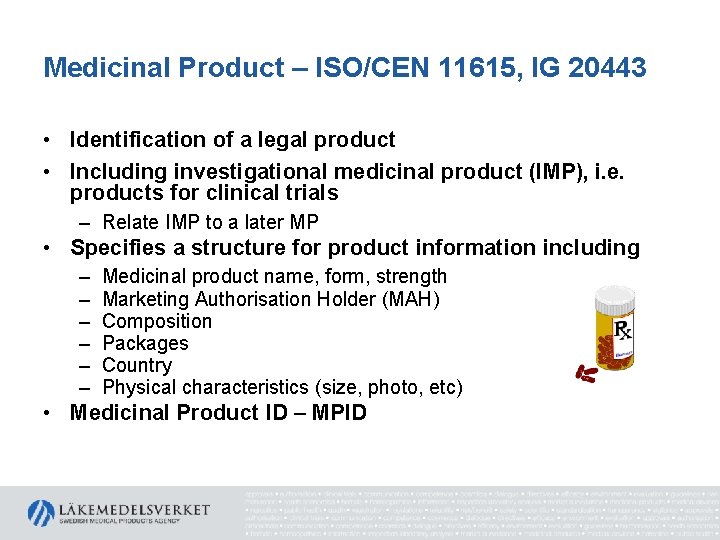
Medicinal Product – ISO/CEN 11615, IG 20443 • Identification of a legal product • Including investigational medicinal product (IMP), i. e. products for clinical trials – Relate IMP to a later MP • Specifies a structure for product information including – – – Medicinal product name, form, strength Marketing Authorisation Holder (MAH) Composition Packages Country Physical characteristics (size, photo, etc) • Medicinal Product ID – MPID

Medicinal Product – ISO/CEN 11615, IG 20443 • Including Medicinal Product Package Identifier (PCID) – package for sale – Dependent on the MPID – Dependent on other properties of the package • packaged item (container)(s) — the type, quantity (items per package), material(s) and alternate material(s); • package component(s) — type, material(s) and alternate material(s); • manufactured item(s) — manufactured dose form, unit of presentation, quantity (items per package). – Changes in these, including the MPID, will affect the PCID • Note: PCID ≠ GTIN for falsified medicines

Medicinal Product – ISO/CEN 11615, IG 20443 • Express the ingredients twice – ”manufactured item” = what is on the shelf in the package – Ingredients when the product is administered to the patient = pharmaceutical product • Explicitly express what substance defines the strength of the product • Including medical devices in the same package

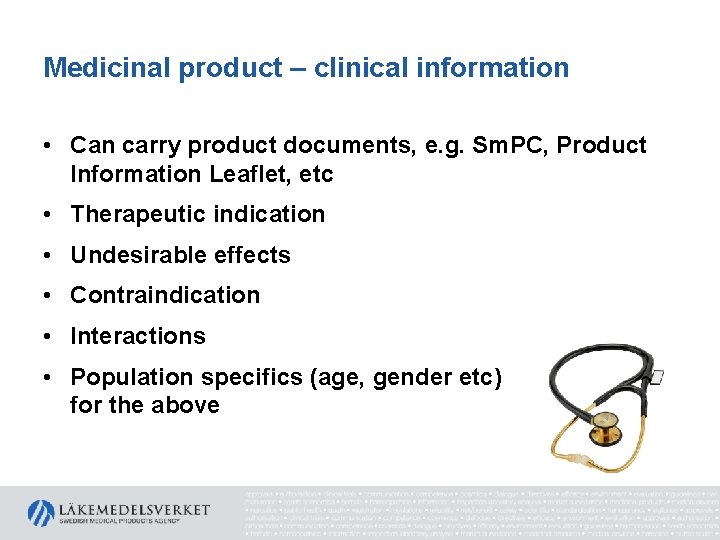
Medicinal product – clinical information • Can carry product documents, e. g. Sm. PC, Product Information Leaflet, etc • Therapeutic indication • Undesirable effects • Contraindication • Interactions • Population specifics (age, gender etc) for the above

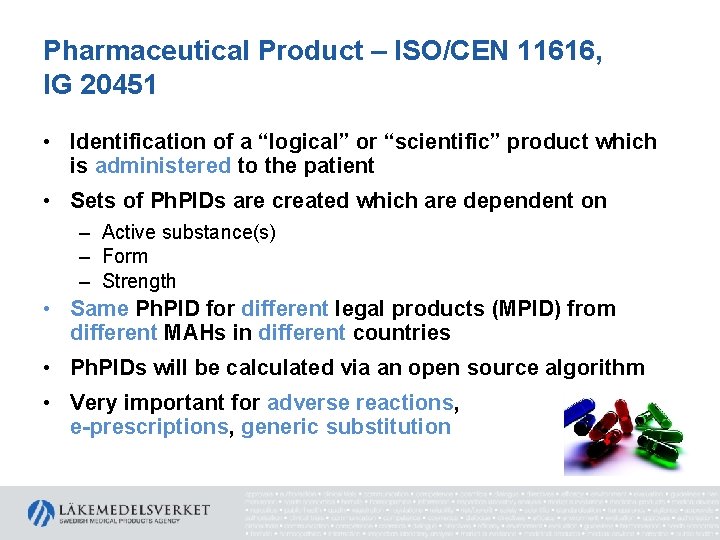
Pharmaceutical Product – ISO/CEN 11616, IG 20451 • Identification of a “logical” or “scientific” product which is administered to the patient • Sets of Ph. PIDs are created which are dependent on – Active substance(s) – Form – Strength • Same Ph. PID for different legal products (MPID) from different MAHs in different countries • Ph. PIDs will be calculated via an open source algorithm • Very important for adverse reactions, e-prescriptions, generic substitution

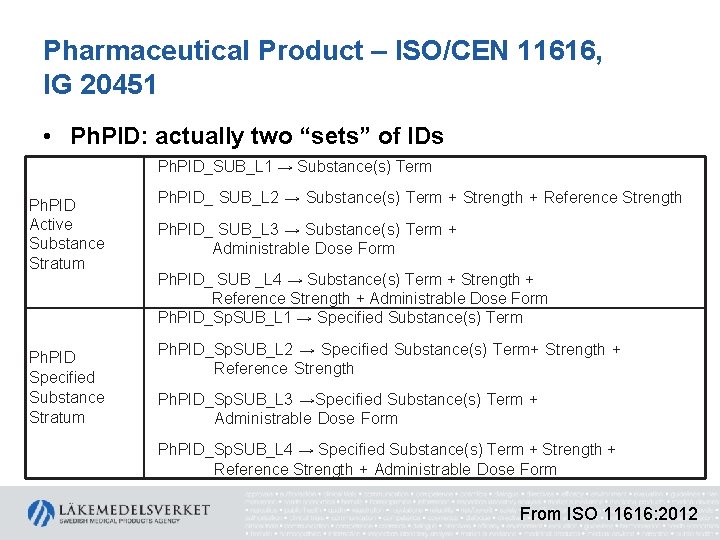
Pharmaceutical Product – ISO/CEN 11616, IG 20451 • Ph. PID: actually two “sets” of IDs Ph. PID Active Substance Stratum Ph. PID Specified Substance Stratum Ph. PID_SUB_L 1 → Substance(s) Term Ph. PID_ SUB_L 2 → Substance(s) Term + Strength + Reference Strength Ph. PID_ SUB_L 3 → Substance(s) Term + Administrable Dose Form Ph. PID_ SUB _L 4 → Substance(s) Term + Strength + Reference Strength + Administrable Dose Form Ph. PID_Sp. SUB_L 1 → Specified Substance(s) Term Ph. PID_Sp. SUB_L 2 → Specified Substance(s) Term+ Strength + Reference Strength Ph. PID_Sp. SUB_L 3 →Specified Substance(s) Term + Administrable Dose Form Ph. PID_Sp. SUB_L 4 → Specified Substance(s) Term + Strength + Reference Strength + Administrable Dose Form From ISO 11616: 2012

Substances – ISO/CEN 11238, IG 19844 • Includes a global substance-ID – Probably based on FDAs UNII code • Most complex of all implementation guides – ca 1000 pages including 10 appendices • All substances, not just active • Software from FDA in testing now: G-SRS from GIn. AS project

Substances – 4 levels • Substance – Single substance – Mixture substance • Specified substance – more specific details about a substance including manufacturing, specifications, etc. – specified substance is always a sub-class to one or more substance(s) • Group 1 • Group 2 • Group 3 • Group 4 (will not be implemented for now)

IDMP Data Pilot • IDMP standards very complex – Implementation guide high level • Need to get good, concrete examples – A group of Agencies, led by Norway and Sweden, are running a data pilot to put real product data into IDMP • AIM: to better understand Medicinal Product and Pharmaceutical Product standards • Publish examples and explanations • Delivery – early 2019 • Input to EMAs EU Implementation Guide

ISO IDMP Adoption Expert Group • ISO-led program to provide examples and explanations – Early stages – has not received official GO from ISO yet