Identifying Unknown Substances Why it is important to

Identifying Unknown Substances

Why it is important to be able to identify unknown substances: 1. To identify a pure substance. 2. To choose the best material to build an object or a machine.

Tungsten 1. Given this unknown sample of metal, how can we identify which element it is? Physical description Freezing point Melting point Density Electrical conductivity Reaction with other substances (Nitric Acid) Oxidation

2. Using the characteristic properties of this metal we can determine its usefulness in making a light bulb. - A high melting point = durable at high temperatures - Electric conductivity = a good conductor - Is not easily oxidized = Doesn’t wear out in the presence of Oxygen.

• Characteristic Properties: Properties that are unique to the given substance only. • Non-characteristic Properties: Properties that are common among different substances.

Properties of Pure Substances • Pure substances (not mixtures) can be described by describing their characteristic properties. • A Characteristic Property is one that helps us to identify a pure substance or the group to which the pure substance belongs. • They can be divided into two categories: – Characteristic physical properties – Characteristic chemical properties

Characteristic Physical Properties • Allow us to identify a substance without changing the nature of the substance in the process. – E. g. Finding the boiling point of water. • This changes the phase of the substance not the nature of it. • No two pure substances boil at the exact same temperature.

The following table contains some examples however there are more including: • Electrical conductivity Heat conductivity Hardness Malleability Magnetism
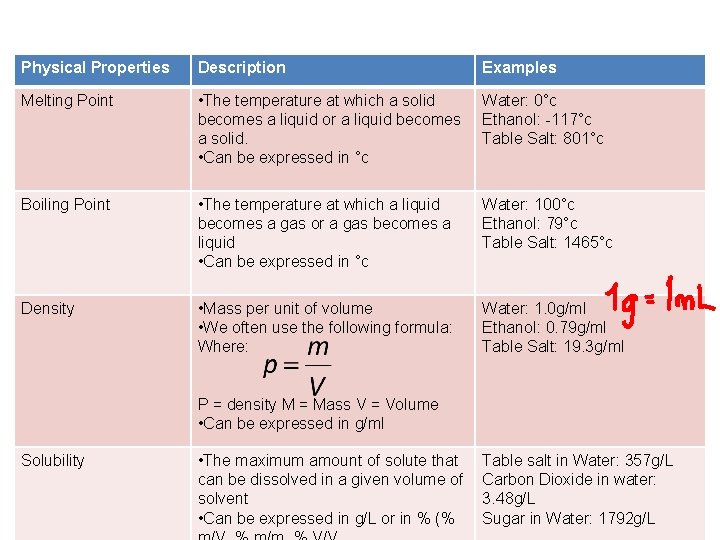
Physical Properties Description Examples Melting Point • The temperature at which a solid becomes a liquid or a liquid becomes a solid. • Can be expressed in °c Water: 0°c Ethanol: -117°c Table Salt: 801°c Boiling Point • The temperature at which a liquid becomes a gas or a gas becomes a liquid • Can be expressed in °c Water: 100°c Ethanol: 79°c Table Salt: 1465°c Density • Mass per unit of volume • We often use the following formula: Where: Water: 1. 0 g/ml Ethanol: 0. 79 g/ml Table Salt: 19. 3 g/ml P = density M = Mass V = Volume • Can be expressed in g/ml Solubility • The maximum amount of solute that can be dissolved in a given volume of solvent • Can be expressed in g/L or in % (% Table salt in Water: 357 g/L Carbon Dioxide in water: 3. 48 g/L Sugar in Water: 1792 g/L
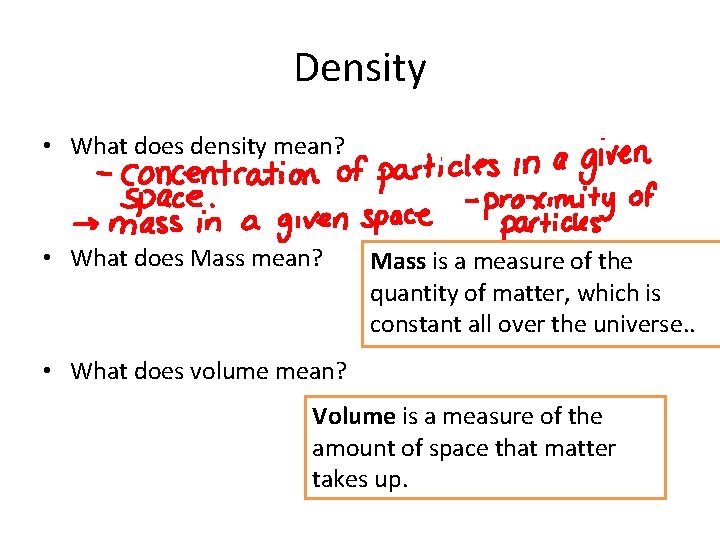
Density • What does density mean? • What does Mass mean? Mass is a measure of the quantity of matter, which is constant all over the universe. . • What does volume mean? Volume is a measure of the amount of space that matter takes up.
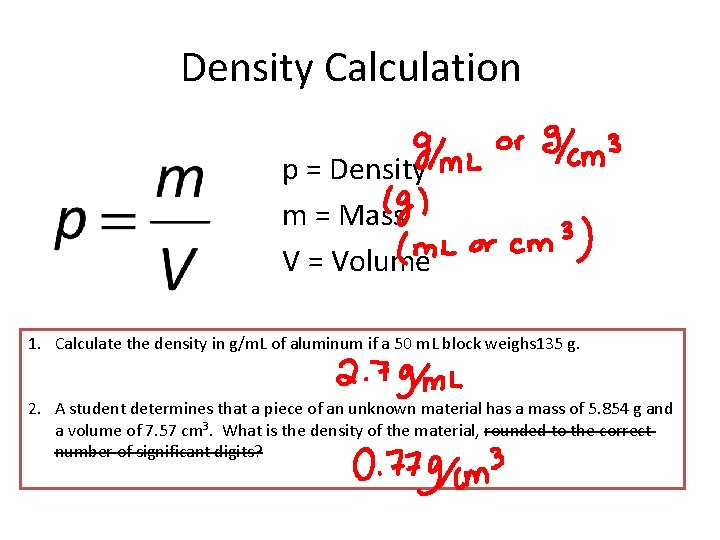
Density Calculation p = Density m = Mass V = Volume 1. Calculate the density in g/m. L of aluminum if a 50 m. L block weighs 135 g. 2. A student determines that a piece of an unknown material has a mass of 5. 854 g and a volume of 7. 57 cm 3. What is the density of the material, rounded to the correct number of significant digits?

WHY DO DIFFERENT SUBSTANCES HAVE DIFFERENT PROPERTIES?
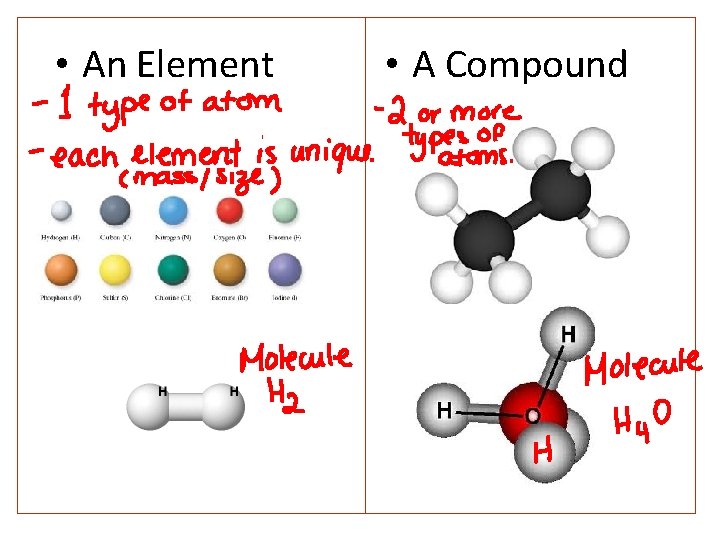
• An Element • A Compound
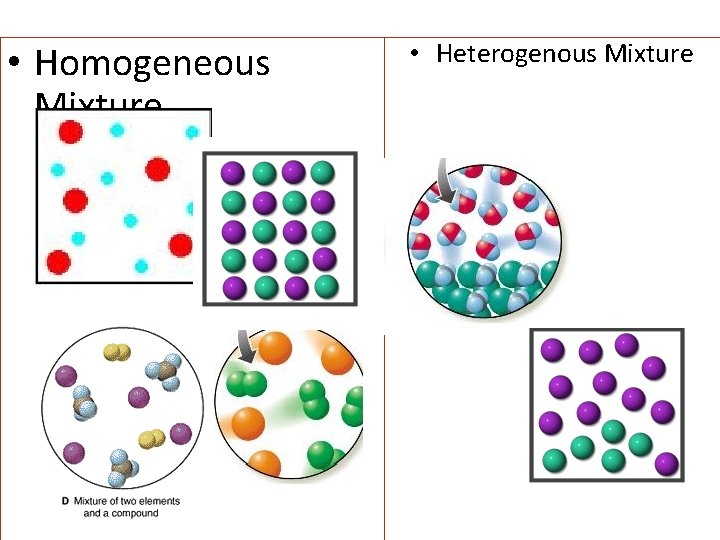
• Homogeneous Mixture • Heterogenous Mixture
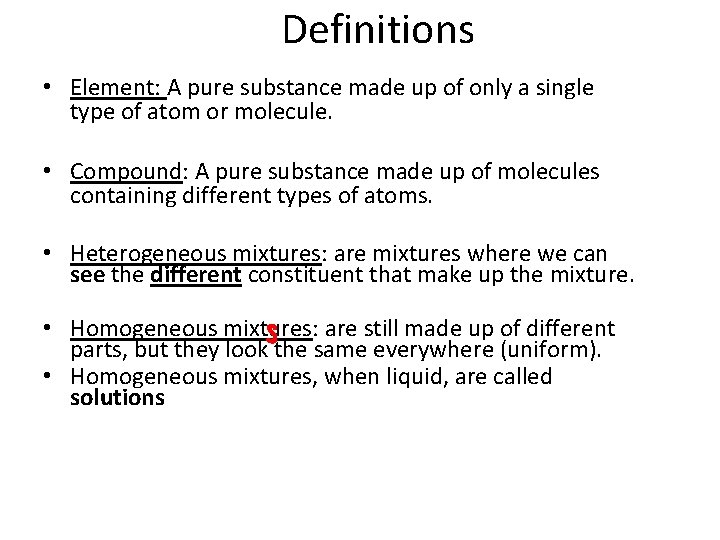
Definitions • Element: A pure substance made up of only a single type of atom or molecule. • Compound: A pure substance made up of molecules containing different types of atoms. • Heterogeneous mixtures: are mixtures where we can see the different constituent that make up the mixture. • Homogeneous mixtures: are still made up of different parts, but they look the same everywhere (uniform). • Homogeneous mixtures, when liquid, are called solutions
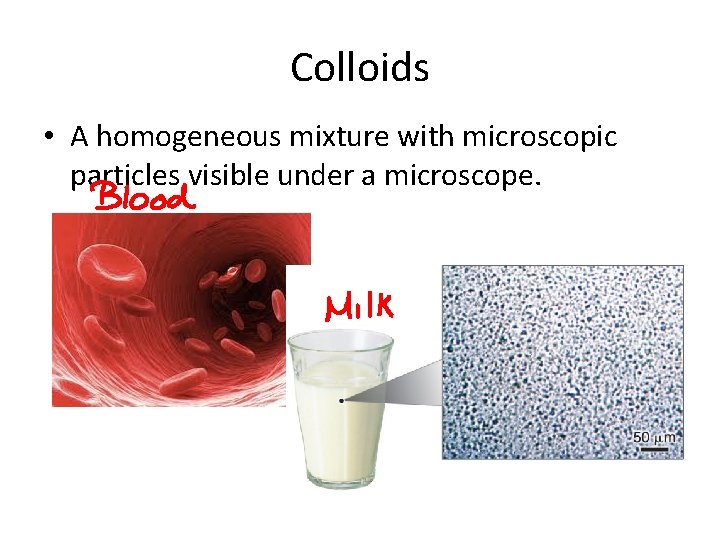
Colloids • A homogeneous mixture with microscopic particles visible under a microscope.

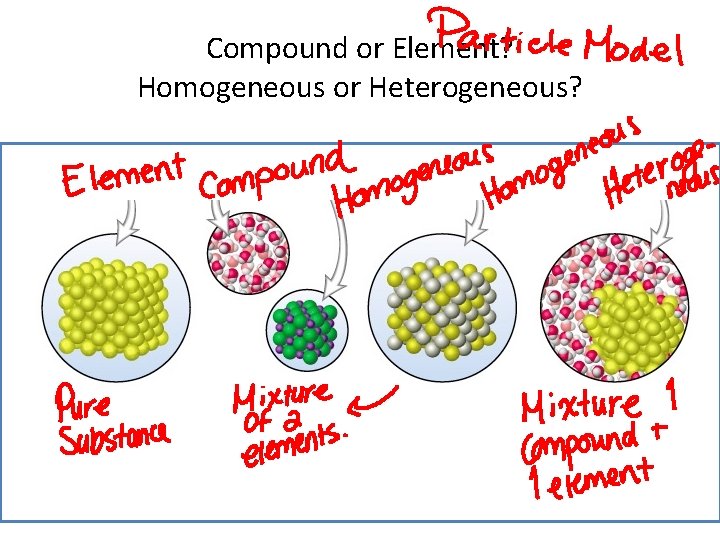
Compound or Element? Homogeneous or Heterogeneous?

Solutions

Solutions • Example: A glass of water with other things dissolved inside, maybe salt. • Each of the substances in that glass of water keeps the original chemical properties. • Can it be separated?
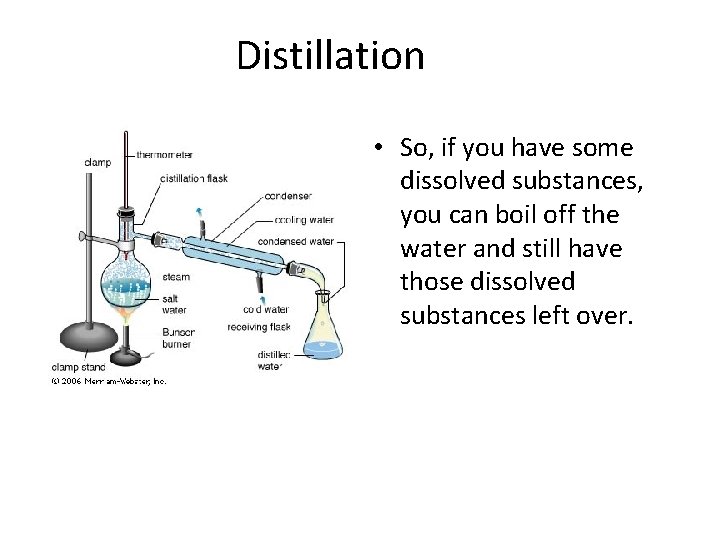
Distillation • So, if you have some dissolved substances, you can boil off the water and still have those dissolved substances left over.
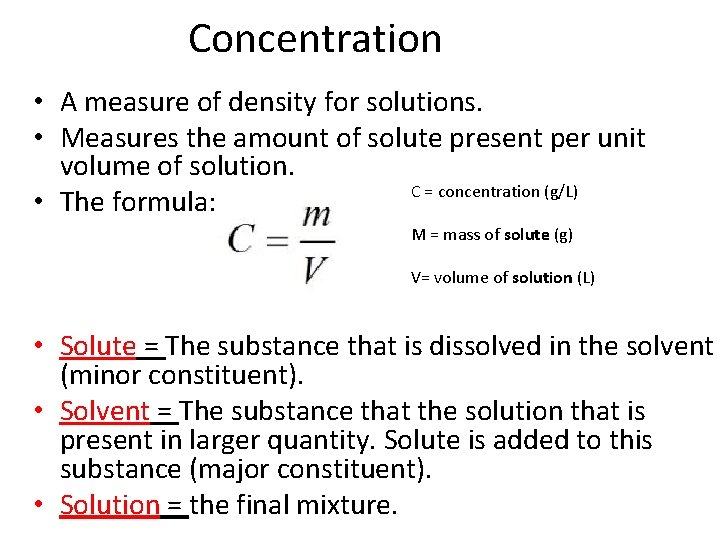
Concentration • A measure of density for solutions. • Measures the amount of solute present per unit volume of solution. C = concentration (g/L) • The formula: M = mass of solute (g) V= volume of solution (L) • Solute = The substance that is dissolved in the solvent (minor constituent). • Solvent = The substance that the solution that is present in larger quantity. Solute is added to this substance (major constituent). • Solution = the final mixture.
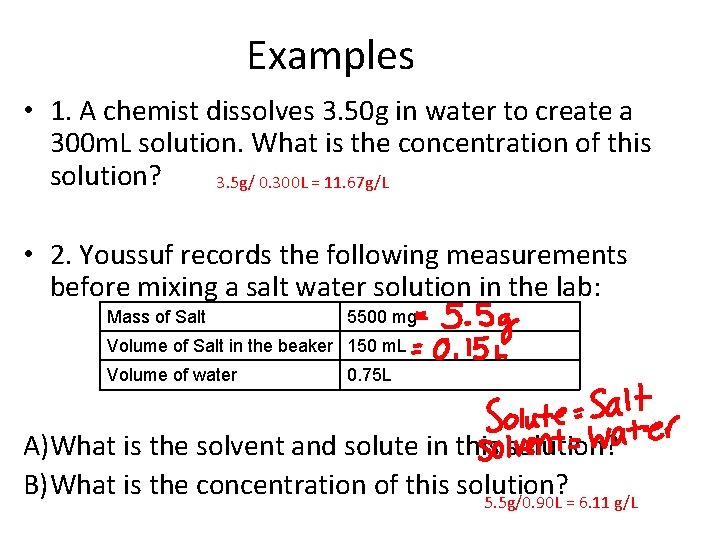
Examples • 1. A chemist dissolves 3. 50 g in water to create a 300 m. L solution. What is the concentration of this solution? 3. 5 g/ 0. 300 L = 11. 67 g/L • 2. Youssuf records the following measurements before mixing a salt water solution in the lab: Mass of Salt 5500 mg Volume of Salt in the beaker 150 m. L Volume of water 0. 75 L A)What is the solvent and solute in this solution? B) What is the concentration of this solution? 5. 5 g/0. 90 L = 6. 11 g/L
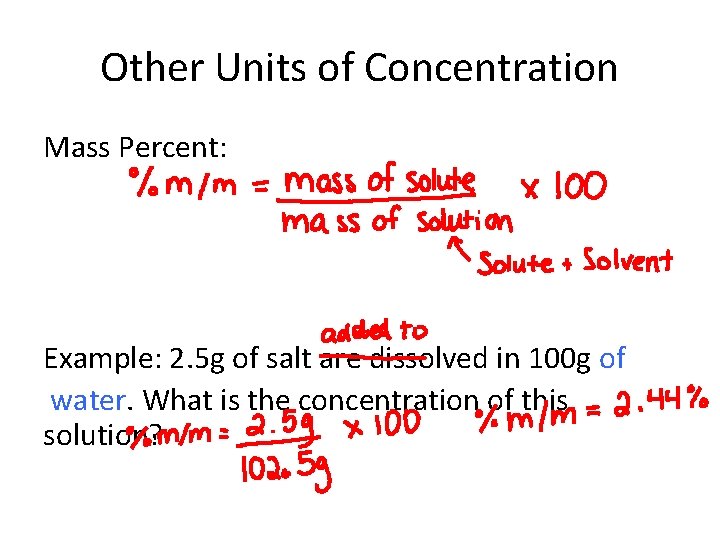
Other Units of Concentration Mass Percent: Example: 2. 5 g of salt are dissolved in 100 g of water. What is the concentration of this solution?

• Mass/Volume Percent: • 2. 5 g salt are dissolved in 500 m. L of a chicken broth solution. What is the concentration of this solution?

• Volume/Volume Percent: • 50 m. L of acetic acid are dissolved in 1 L of vinegar. What is the concentration of this solution?

Note for answering concentration questions • When something is dissolved in a solvent. – Ex: salt dissolved in water • You need to add the solute and solvent!!! • When you are dissolving in a solution. – Ex: acid dissolved in a vinegar solution. • You do not need to add anything before finding your concentration!!!

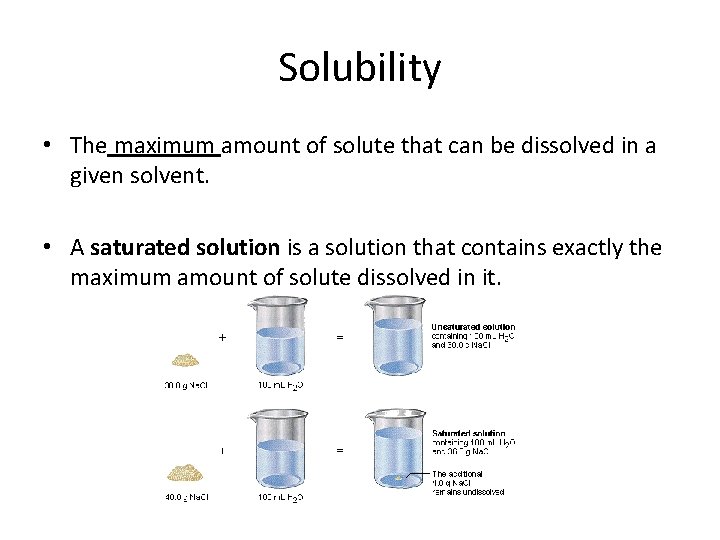
Solubility • The maximum amount of solute that can be dissolved in a given solvent. • A saturated solution is a solution that contains exactly the maximum amount of solute dissolved in it.

DEMO!
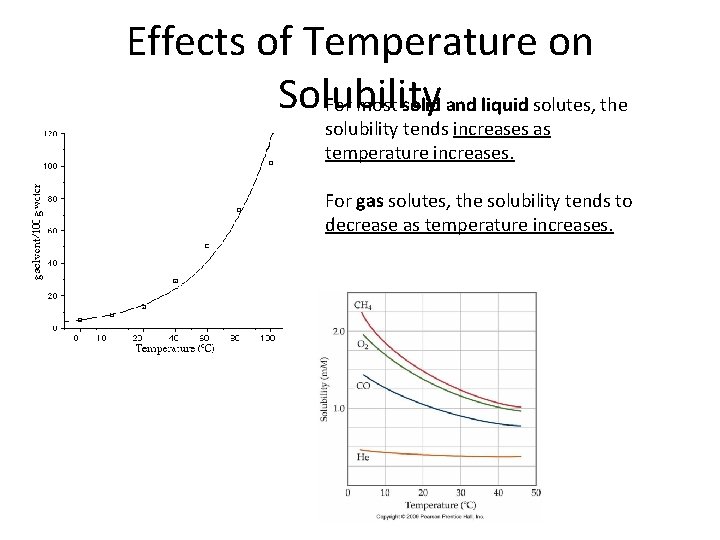
Effects of Temperature on Solubility For most solid and liquid solutes, the solubility tends increases as temperature increases. For gas solutes, the solubility tends to decrease as temperature increases.
- Slides: 31