IDENTIFYING ACIDS AND BASES USING HOMEMADE INDICATORS Moving

IDENTIFYING ACIDS AND BASES USING HOME-MADE INDICATORS Moving Forward With Key Competences

THE AIM OF THE EXPERIMENT To demonstrate that experiment can also be carried outside the labs

REQUIRED MATERIALS 2 big beakers Purple cabbage 4. 5 Glass stirror 10 little beakers (100 ml) Vinegar Lemon juice Dishwashing liquid

Laundry detergent Laundry soda Bleach Mineral water 1 lt boiled water

THE PROCEDURE Put a few leaves of cabbage into a big beaker. Add some boiling water. We wait for 15 minutes. And the blue indicator is ready.

INDICATOR Organic compound that changes colour according to p. H of the solution. We put that blue liquid into 100 ml beakers. Then we add vinegar, diswashing liquid, laundry detergent, lemon juice, laundry soda, bleach or mineral water to each beaker.

THE RESULT Functioning as an indicator, in the purple cabbage enables us to observe change of colour according to p. H of the substance we add to it.
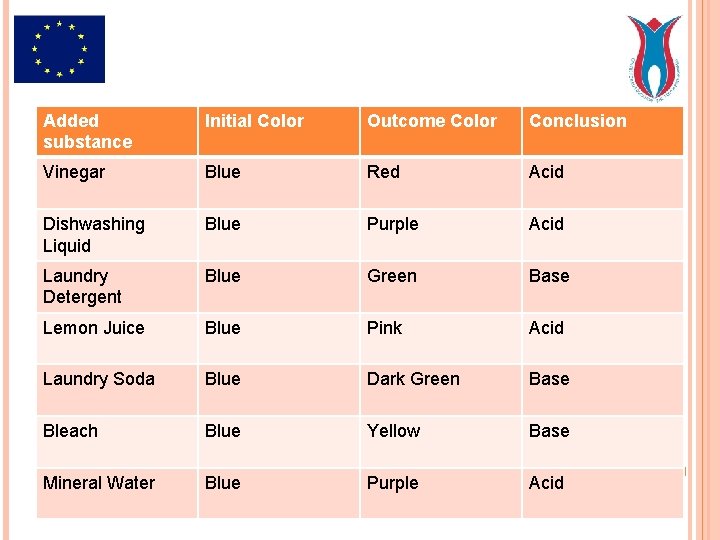
Added substance Initial Color Outcome Color Conclusion Vinegar Blue Red Acid Dishwashing Liquid Blue Purple Acid Laundry Detergent Blue Green Base Lemon Juice Blue Pink Acid Laundry Soda Blue Dark Green Base Bleach Blue Yellow Base Mineral Water Blue Purple Acid

THE RESULTS

- Slides: 10