Identify major stages of the process Location of



![� Why use excess when its not needed? �[ATP] activates/inactivates control enzyme (phosphofructokinase) �Enzyme � Why use excess when its not needed? �[ATP] activates/inactivates control enzyme (phosphofructokinase) �Enzyme](https://slidetodoc.com/presentation_image_h2/a3c4b0e866b05ea08a042e1358184dc8/image-12.jpg)




- Slides: 39

� Identify major stages of the process � Location of each stage �Describe structures �Illustrate with simple diagrams �Indicate how ingredients are acquired/products released � Factors that affect the rate of respiration

I. III. IV. V. VI. Group learning: what is respiration? General information & description. Become an expert on one part of the process Educate your group members Collaborate and sync up the entire process Make connections & compare The BIG picture! Connect with photosynthesis 1. Anaerobic respiration: Compare energy efficiency with aerobic respiration 2. Glycolysis 3. Krebs Cycle (aka Citric Acid Cycle) 4. Electron Transport Chain 5. Factors that affect the rate of respiration

� Online text & animations � Class notes linked to Mrs. De. Nicola’s website � Animations on “AP Bio Links” page � Chapter 7 Review questions � Energy packets


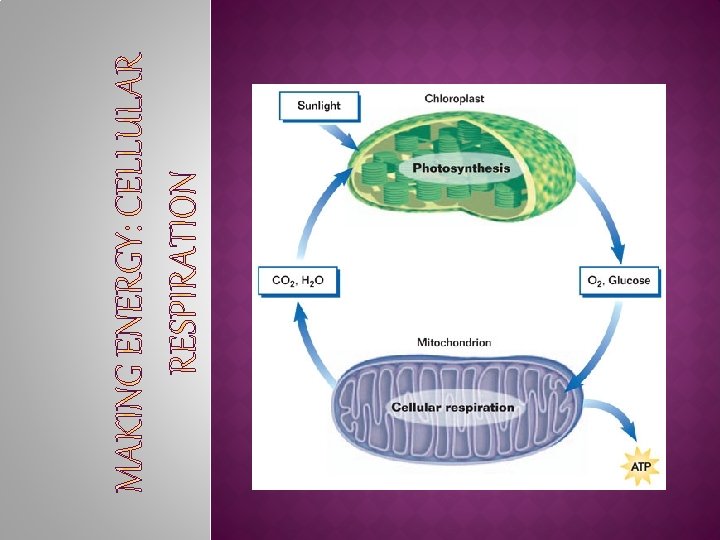
**Glucose made as a byproduct of photosynthetic reactions Harvesting stored energy… � Energy stored in organic molecules �carbohydrates, � Heterotrophs �digestive fats, proteins eat food results… raw materials for synthesis fuels for energy �controlled release of energy �“burning” fuels occurs in series of step-by-step enzyme-controlled reactions

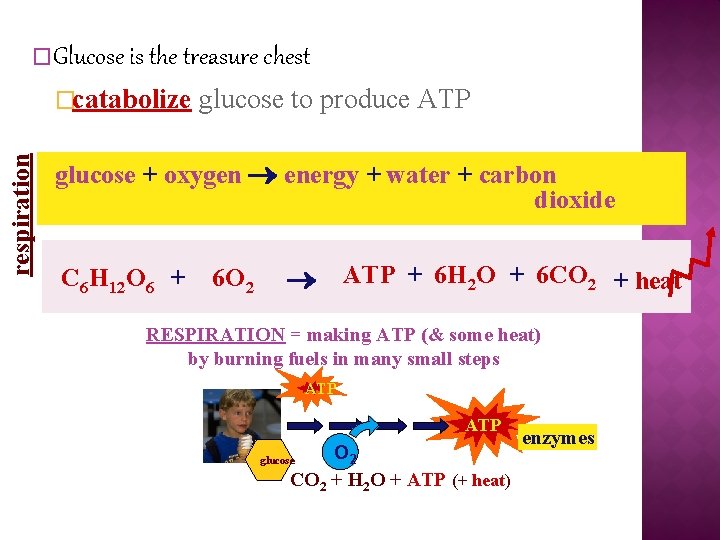
�Glucose is the treasure chest respiration �catabolize glucose to produce ATP glucose + oxygen energy + water + carbon dioxide C 6 H 12 O 6 + 6 O 2 ATP + 6 H 2 O + 6 CO 2 + heat RESPIRATION = making ATP (& some heat) by burning fuels in many small steps ATP O 2 glucose CO 2 + H 2 O + ATP (+ heat) enzymes

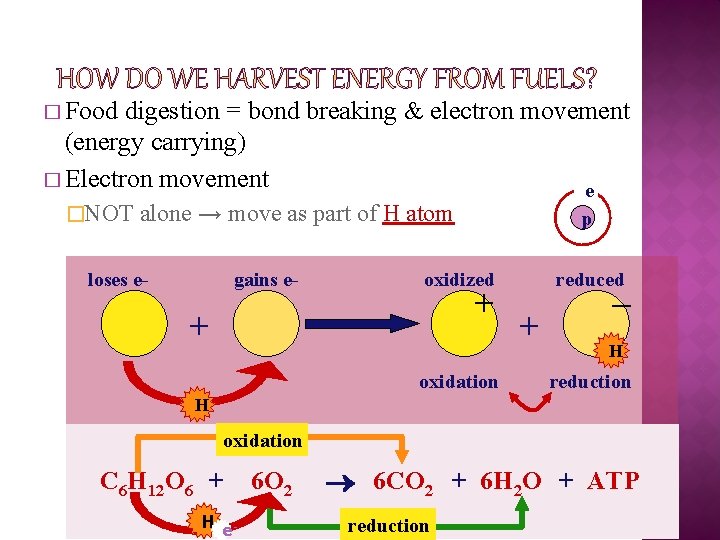
� Food digestion = bond breaking & electron movement (energy carrying) � Electron movement e �NOT alone → move as part of H atom loses e- gains e- p oxidized + + oxidation reduced + – H reduction H oxidation C 6 H 12 O 6 + H e- 6 O 2 6 CO 2 + 6 H 2 O + ATP reduction

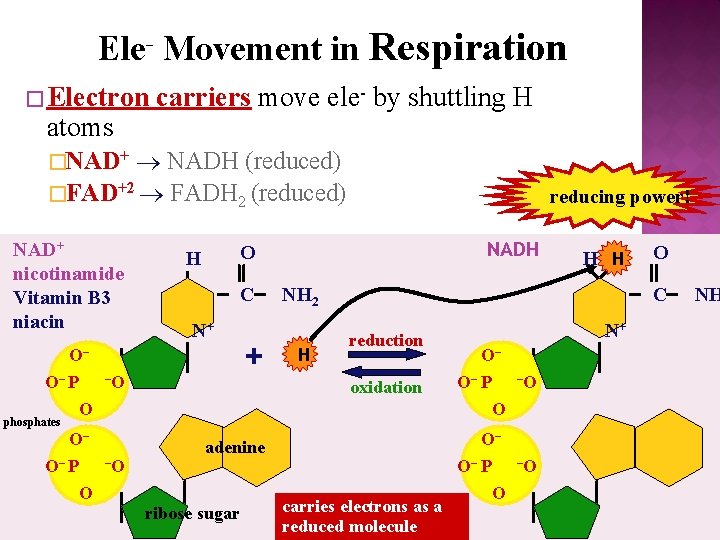
Ele- Movement in Respiration � Electron atoms carriers move ele- by shuttling H NADH (reduced) �FAD+2 FADH 2 (reduced) �NAD+ nicotinamide Vitamin B 3 niacin reducing power! NADH O H C N+ O– O– P – O O + NH 2 H –O reduction oxidation N+ O– O– P –O O O– O– P adenine O ribose sugar O C phosphates O– O– P H H carries electrons as a reduced molecule O –O NH

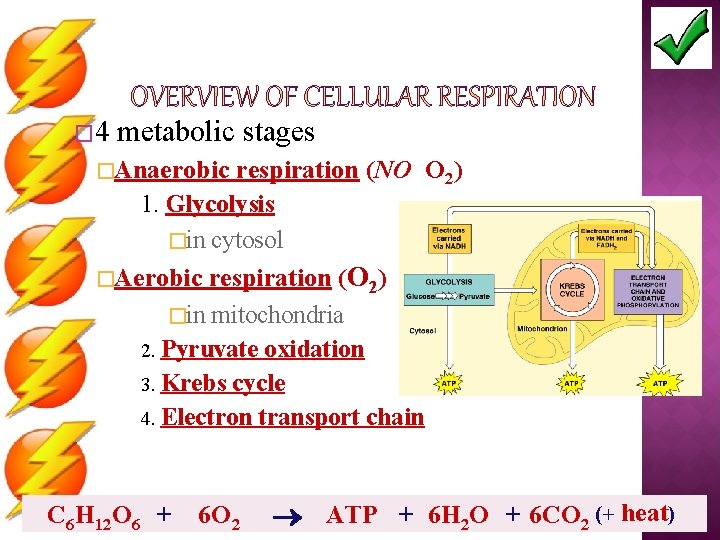
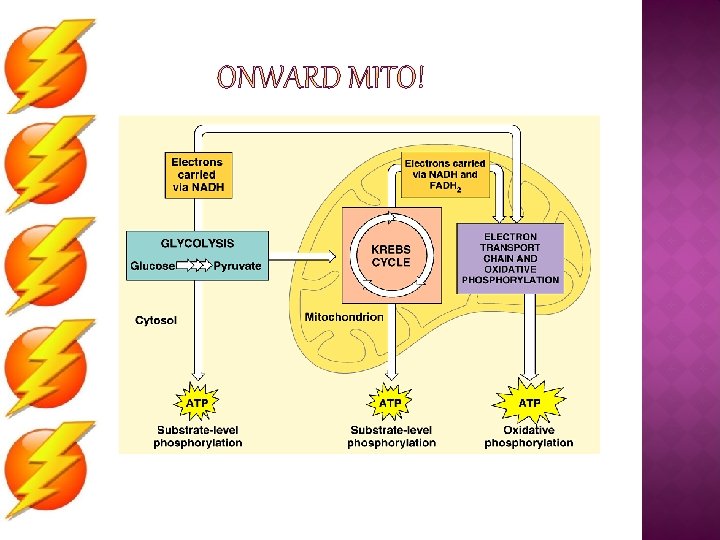
� 4 metabolic stages �Anaerobic respiration (NO O 2) 1. Glycolysis �in cytosol �Aerobic respiration (O 2) �in mitochondria 2. Pyruvate oxidation 3. Krebs cycle 4. Electron transport chain C 6 H 12 O 6 + 6 O 2 ATP + 6 H 2 O + 6 CO 2 (+ heat)

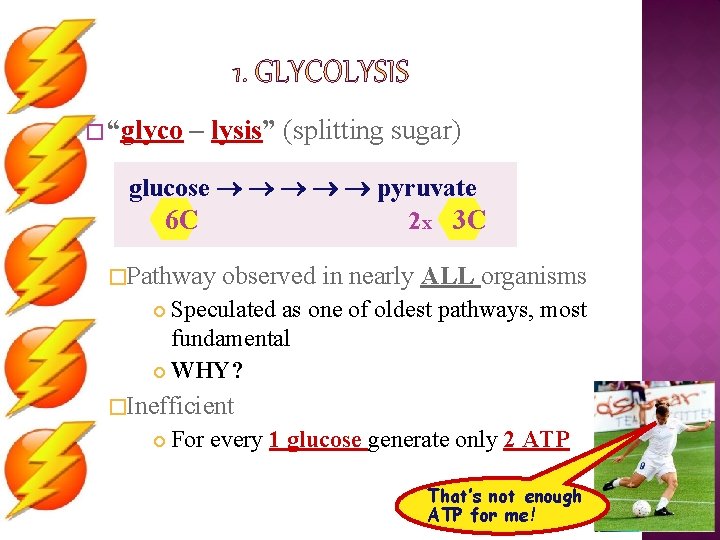
� “glyco – lysis” (splitting sugar) glucose pyruvate 2 x 3 C 6 C �Pathway observed in nearly ALL organisms Speculated as one of oldest pathways, most fundamental WHY? �Inefficient For every 1 glucose generate only 2 ATP That’s not enough ATP for me!

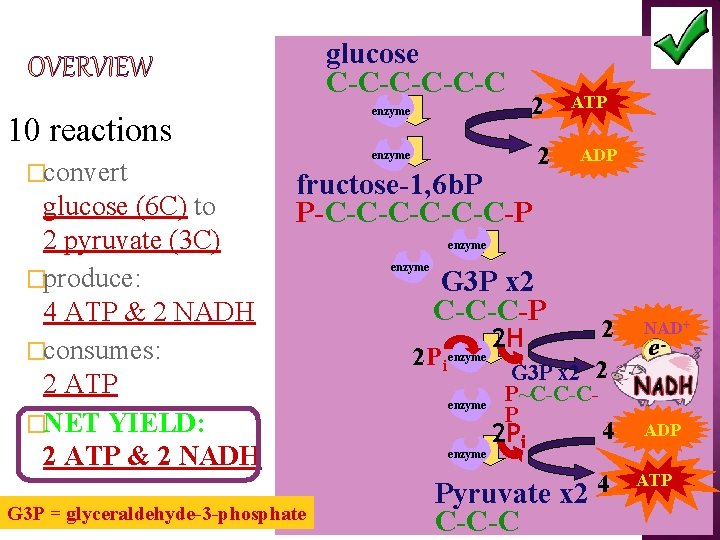
glucose C-C-C-C enzyme 10 reactions �convert glucose (6 C) to 2 pyruvate (3 C) �produce: 4 ATP & 2 NADH �consumes: 2 ATP �NET YIELD: 2 ATP & 2 NADH 2 enzyme fructose-1, 6 b. P P-C-C-C-P G 3 P = glyceraldehyde-3 -phosphate 2 ATP ADP enzyme G 3 P x 2 C-C-C-P 2 Pi enzyme 2 H 2 G 3 P x 2 2 P~C-C-CP 2 Pi 4 Pyruvate x 2 4 C-C-C NAD+ ADP ATP
![Why use excess when its not needed ATP activatesinactivates control enzyme phosphofructokinase Enzyme � Why use excess when its not needed? �[ATP] activates/inactivates control enzyme (phosphofructokinase) �Enzyme](https://slidetodoc.com/presentation_image_h2/a3c4b0e866b05ea08a042e1358184dc8/image-12.jpg)
� Why use excess when its not needed? �[ATP] activates/inactivates control enzyme (phosphofructokinase) �Enzyme used to make phosphorylated glucose �Allosteric � 2 active sites 1. forms phosphorylated glucose 2. conformation change inactivate regulation!!!

Is this enough to support life? � Not a lot of energy… �for 1 billon years+ life on Earth survived this way no O 2 = slow growth, slow reproduction only harvest 3. 5% of energy stored in glucose �more carbons to strip off = more energy to harvest O 2 O 2 present O 2 O 2 Onto the Krebs Cycle!!!


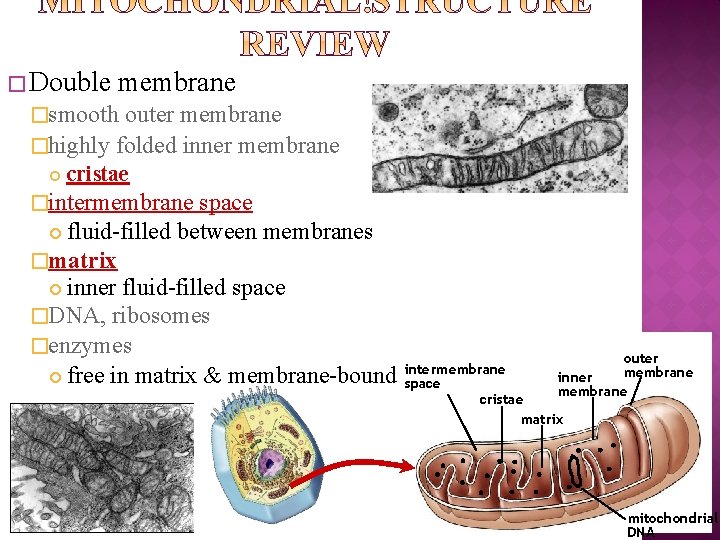
� Double membrane �smooth outer membrane �highly folded inner membrane cristae �intermembrane space fluid-filled between membranes �matrix inner fluid-filled space �DNA, ribosomes �enzymes free in matrix & membrane-bound outer intermembrane inner space membrane cristae matrix mitochondrial DNA

Prepping for Krebs: formation of Acetyl Co. A NAD+ Pyruvate C-C-C 2 x reduction Coenzyme A CO 2 Acetyl Co. A C-C oxidation [ Yield = 2 C sugar + NADH + CO ] (Acetyl Co. A) 2

1937 | 1953 �aka Citric Acid Cycle �in mitochondrial matrix � 8 step pathway each catalyzed by specific enzyme step-wise catabolism of 6 C citrate molecule (stripping out the carbons) �Appeared later than glycolysis – WHY? Hans Krebs 1900 -1981

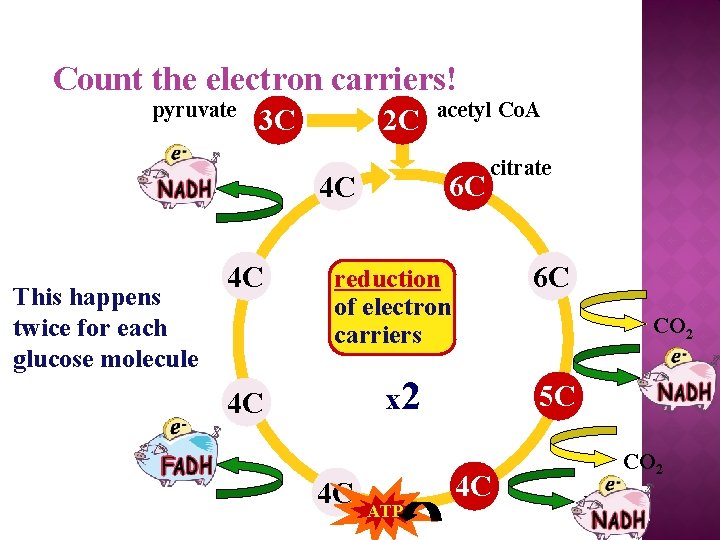
Count the carbons! pyruvate 3 C 2 C acetyl Co. A 6 C 4 C This happens twice for each glucose molecule **Process regulated by + and – feedback control by [ATP]!!!** 4 C citrate 6 C oxidation of sugars CO 2 x 2 4 C 4 C 5 C 4 C CO 2

Count the electron carriers! pyruvate 3 C 4 C citrate 6 C reduction of electron carriers CO 2 x 2 4 C FADH 2 acetyl Co. A 6 C 4 C NADH This happens twice for each glucose molecule 2 C 4 C ATP NADH 5 C 4 C CO 2 NADH

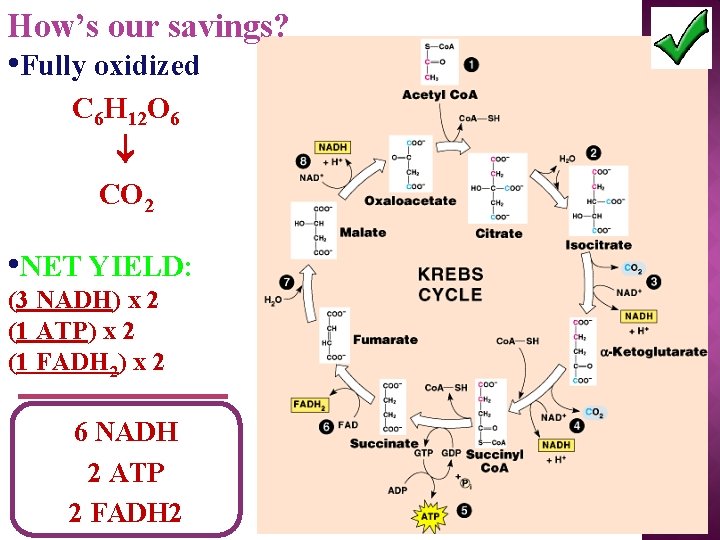
How’s our savings? • Fully oxidized C 6 H 12 O 6 CO 2 • NET YIELD: (3 NADH) x 2 (1 ATP) x 2 (1 FADH 2) x 2 6 NADH 2 ATP 2 FADH 2

LET’S RECAP… 2 ATP �Kreb’s cycle 2 ATP �Glycolysis lot a d e I ne TP! A e r mo �Life takes a lot of energy to run, need to extract more energy than 4 ATP! �Fun Fact!!! A working muscle recycles over 10 million ATPs per second

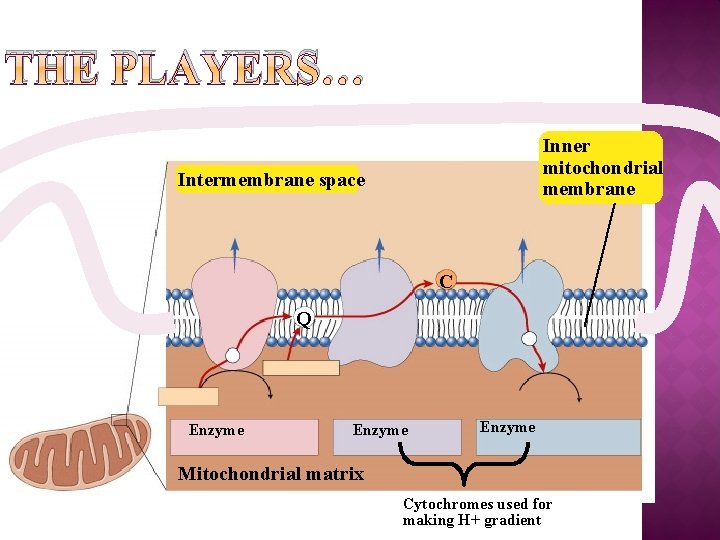
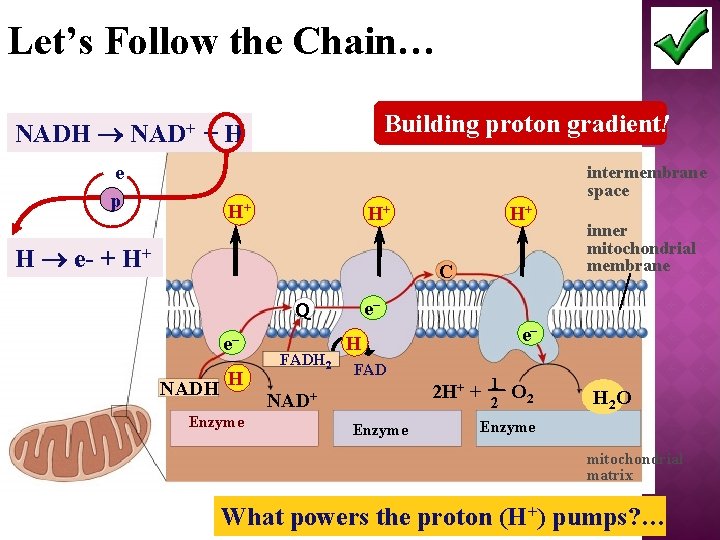
�Proteins built into inner mitochondrial membrane along cristae transport proteins & enzymes �In presence of O 2 �Ele- shuttled (by NADH & FADH 2)down ETC pump H+ to create H+ gradient → chemiosmosis!!! �yields ~36 ATP from 1 glucose!

THE PLAYERS… Inner mitochondrial membrane Intermembrane space C Q Enzyme Mitochondrial matrix Cytochromes used for making H+ gradient

Let’s Follow the Chain… Building proton gradient! NADH NAD+ + H e p intermembrane space H+ H+ H e- + H+ H+ C e– Q e– NADH H Enzyme FADH 2 inner mitochondrial membrane e– H FAD 2 H+ + NAD+ Enzyme 1 2 O 2 H 2 O Enzyme mitochondrial matrix What powers the proton (H+) pumps? …

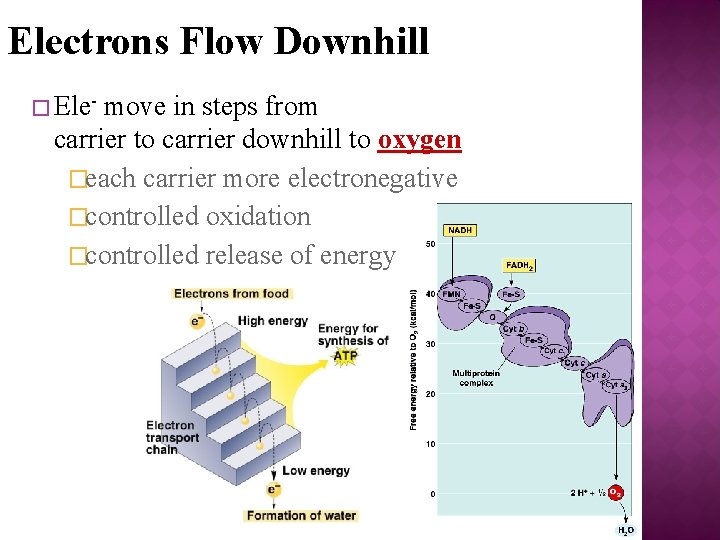
Electrons Flow Downhill � Ele- move in steps from carrier to carrier downhill to oxygen �each carrier more electronegative �controlled oxidation �controlled release of energy

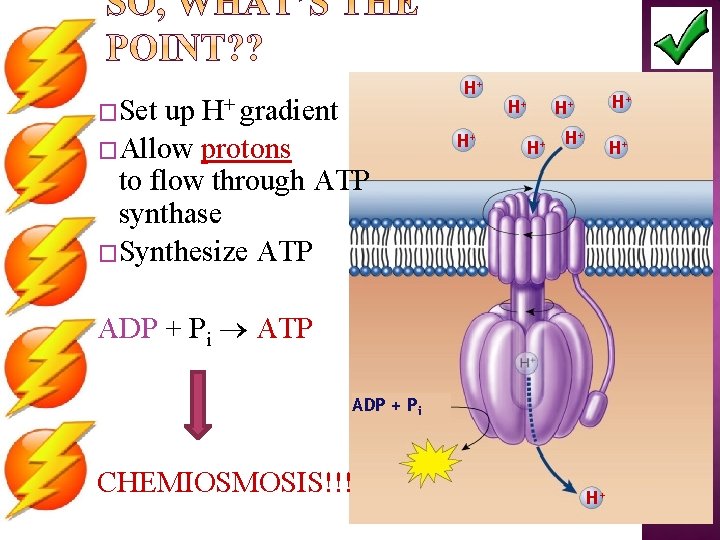
�Set H+ gradient up �Allow protons to flow through ATP synthase �Synthesize ATP H+ H+ ADP + Pi ATP ADP + Pi CHEMIOSMOSIS!!! H+

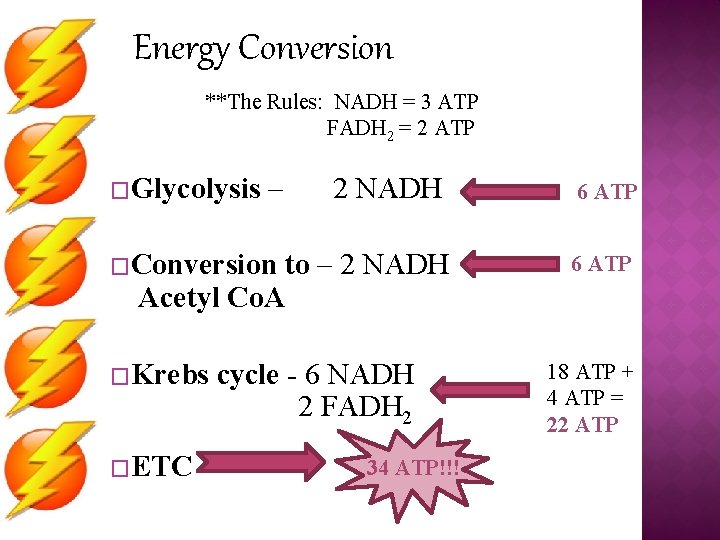
Energy Conversion **The Rules: NADH = 3 ATP FADH 2 = 2 ATP �Glycolysis – 2 NADH �Conversion to – 2 NADH Acetyl Co. A �Krebs �ETC cycle - 6 NADH 2 FADH 2 34 ATP!!! 6 ATP 18 ATP + 4 ATP = 22 ATP

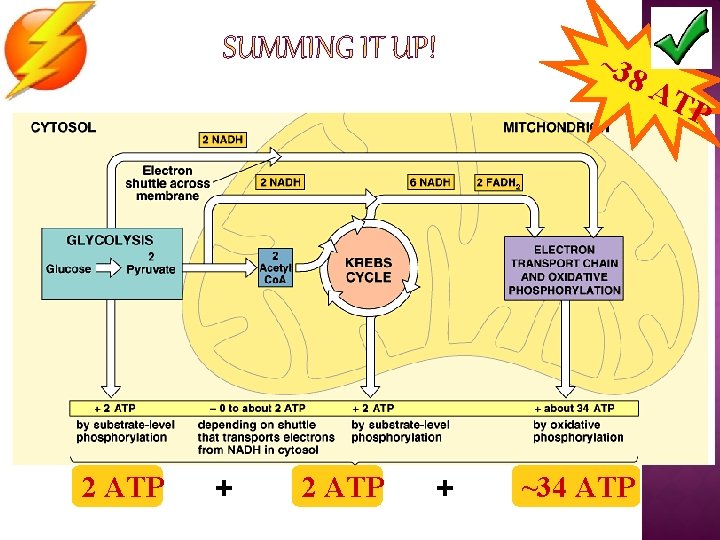
~38 2 ATP + ~34 ATP AT P


proteins amino acids hydrolysis waste O H H | || C—OH N —C— H | R amino group = Waste, excreted as ammonia, urea, or uric acid glycolysis Krebs cycle 2 C sugar = carbon skeleton = enters glycolysis or Krebs cycle

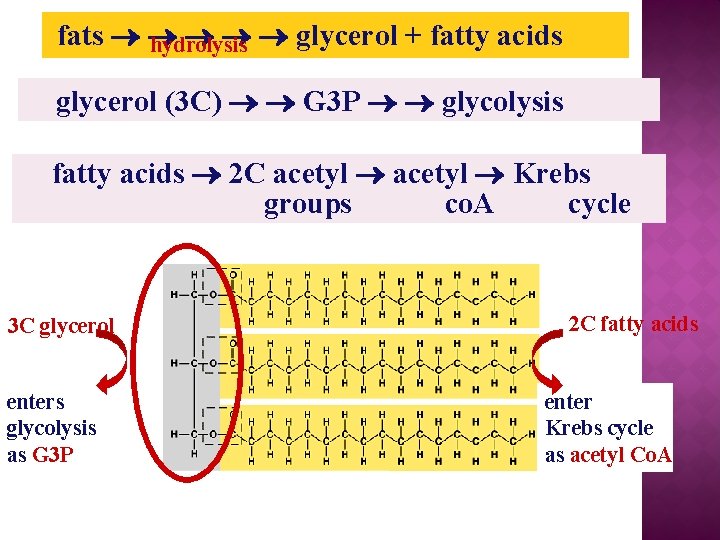
fats glycerol + fatty acids hydrolysis glycerol (3 C) G 3 P glycolysis fatty acids 2 C acetyl Krebs groups co. A cycle 3 C glycerol enters glycolysis as G 3 P 2 C fatty acids enter Krebs cycle as acetyl Co. A

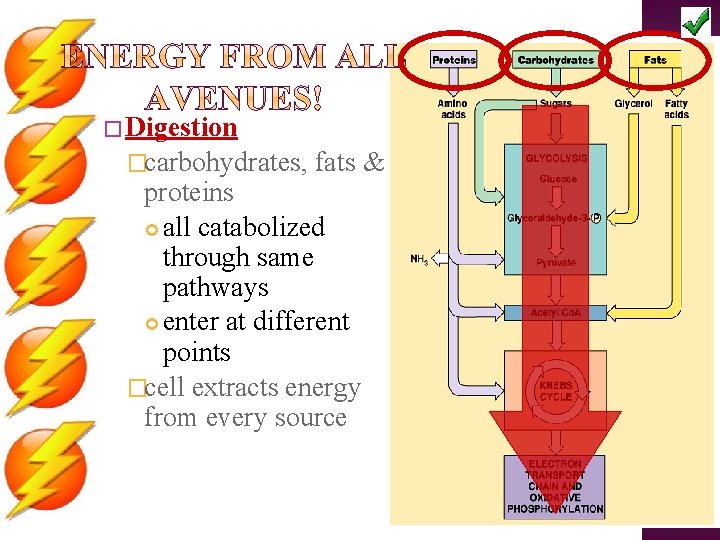
� Digestion �carbohydrates, fats & proteins all catabolized through same pathways enter at different points �cell extracts energy from every source

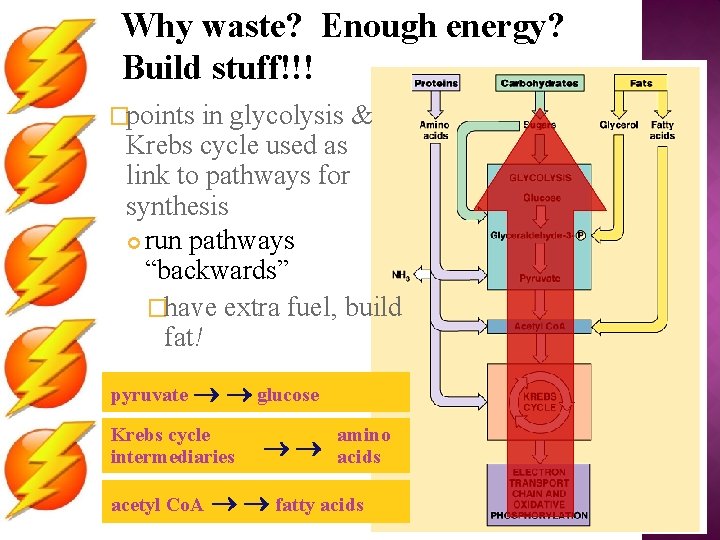
Why waste? Enough energy? Build stuff!!! �points in glycolysis & Krebs cycle used as link to pathways for synthesis run pathways “backwards” �have extra fuel, build fat! pyruvate glucose Krebs cycle intermediaries acetyl Co. A amino acids fatty acids

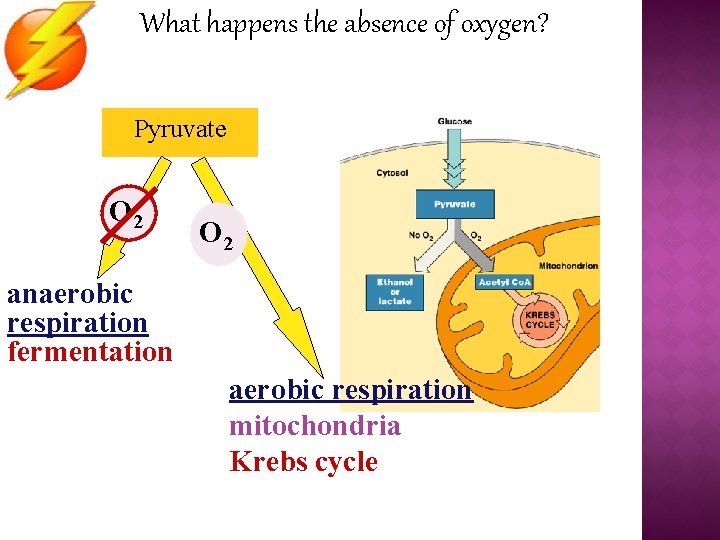
What happens the absence of oxygen? Pyruvate O 2 anaerobic respiration fermentation aerobic respiration mitochondria Krebs cycle

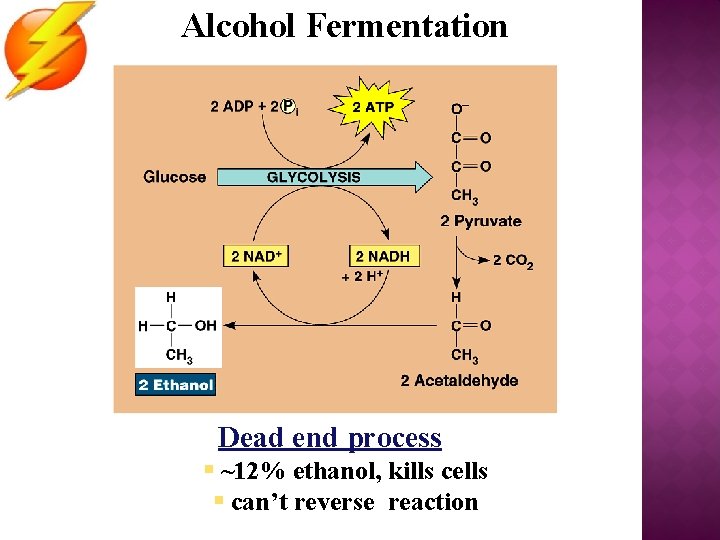
Alcohol Fermentation Dead end process § ~12% ethanol, kills cells § can’t reverse reaction

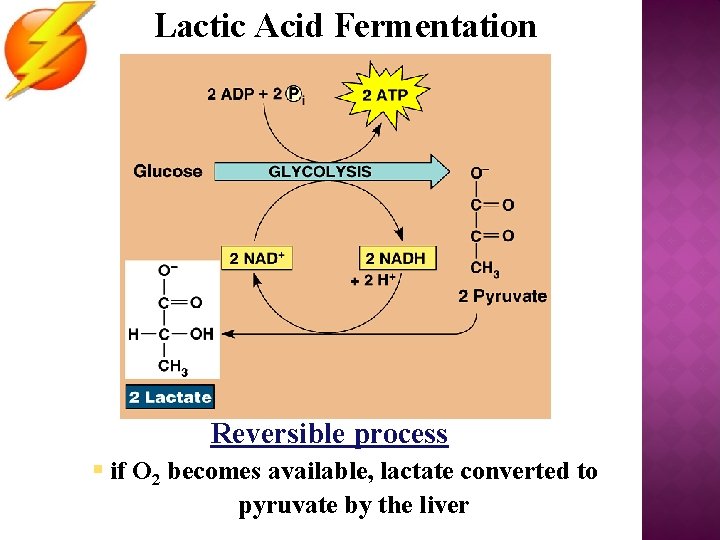
Lactic Acid Fermentation Reversible process § if O 2 becomes available, lactate converted to pyruvate by the liver

recycle NADH

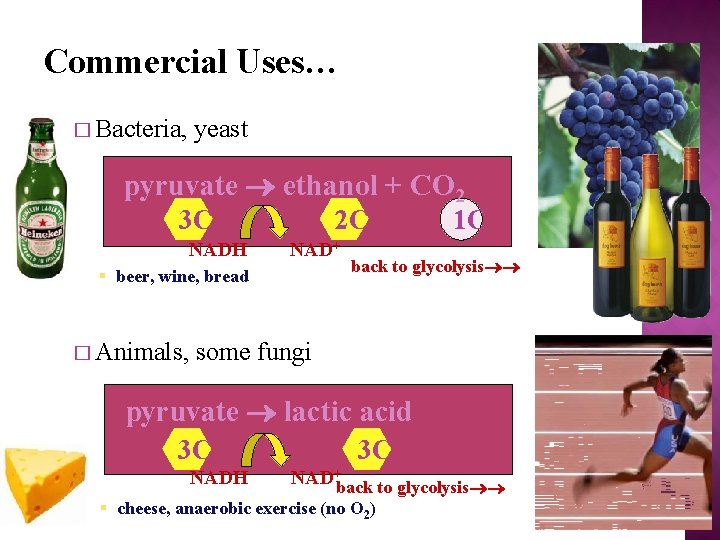
Commercial Uses… � Bacteria, yeast pyruvate ethanol + CO 2 3 C NADH 2 C NAD+ § beer, wine, bread � Animals, 1 C back to glycolysis some fungi pyruvate lactic acid 3 C NADH 3 C NAD+back to glycolysis § cheese, anaerobic exercise (no O 2)

� Cell Respiration with Hank � ETC � ATP Synthase