IDENTIFICATION OF PLASTICS IDENTIFICATION When working with plastics






































- Slides: 68

IDENTIFICATION OF PLASTICS

IDENTIFICATION When working with plastics there is often a need to identify which particular plastic material has been used for a given product. This is essential to get an idea of the cost and likely properties of the product. The identification of plastics is generally very difficult due to: • • • The wide range of basic polymers that is available for use. The wide range of additives that can be used to modify the properties of the basic polymer. The wide range of mixtures or compounds of polymers that can be manufactured to get the required properties. Despite this there are some simple tests that can be carried out to get a basic idea of the possible base polymer used for the manufacture of any given product. Corporate Training & Planning 2

• Introduction • Simple method – cutting test – hot wire penetration test – flotation test – bending test – dropping test • Burning test • Pyrolysis test • Specific gravity test • Solubility test • Softening and Melting point • Elemental Analysis • Confirmation test Corporate Training & Planning 3

IDENTIFICATION BY SIMPLE METHOD • Stage 1: Look at the sample This will give you a lot of information. The colour of the plastic will give you some information. Some polymers have restricted colour ranges, particularly thermosetting types. Others tend to be glossier in colour (polypropylene), whereas some are both glossy and glassy (the acrylics). • Stage 2: Feel the sample After you have carried out the tests in the is series a few times you will start to get the feel for various plastics. The polyolefins have a very distinctive feel and you can generally tell if it is one of them. The presence of glass fibre or other reinforcement materials can alter the feel and stiffness of the sample but you can sometimes tell by the feel if there is reinforcement present. • Stage 3: Cut a thin sliver from the edge of the sample. Follow the links to the appropriate page. The first test is to cut a small sliver off the sample. This tells you a lot about the type of plastic you are trying to identify Corporate Training & Planning 4

CUTTING TESTS • If a shaving can be pared off with knife, it may be a thermoplastic. Note: PMMA and Polystyrene are brittle and difficult to pare • If the material is rigid and will not pare off instead flakes of powders, it may probably a thermoset plastic. • Scuff the sample with your fingernail. Results – Scuffs with fingernail - Urea formaldehyde resin. – Does not scuff with fingernail - Melamine formaldehyde resin. Corporate Training & Planning 5

HOT ROD PENETRATION TEST • Heat an electronic soldering iron to red hot and press against the unknown sample. • If the plastic material softens, and the rod penetrates the sample is thermoplastic. • If the plastic material does not soften and the rod does not penetrate, the sample is thermoset plastic. Corporate Training & Planning 6

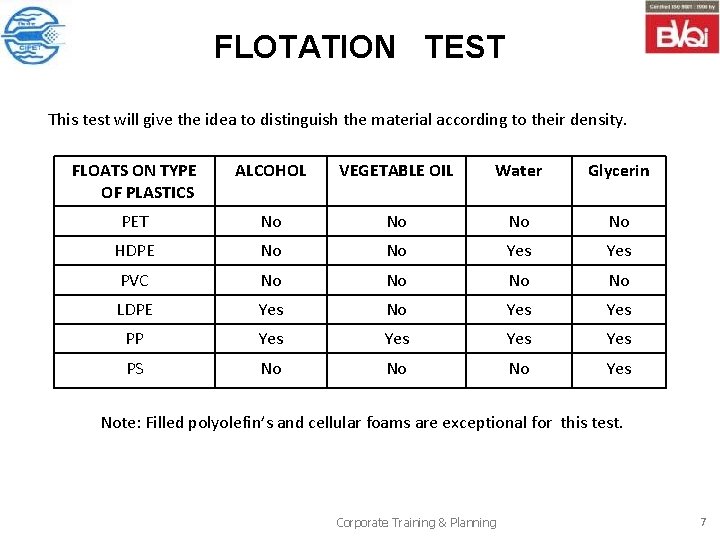
FLOTATION TEST This test will give the idea to distinguish the material according to their density. FLOATS ON TYPE OF PLASTICS ALCOHOL VEGETABLE OIL Water Glycerin PET No No HDPE No No Yes PVC No No LDPE Yes No Yes PP Yes Yes PS No No No Yes Note: Filled polyolefin’s and cellular foams are exceptional for this test. Corporate Training & Planning 7

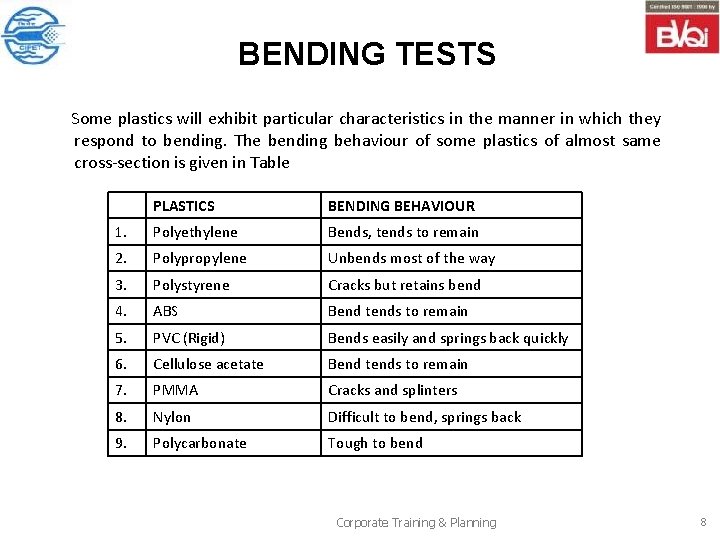
BENDING TESTS Some plastics will exhibit particular characteristics in the manner in which they respond to bending. The bending behaviour of some plastics of almost same cross-section is given in Table PLASTICS BENDING BEHAVIOUR 1. Polyethylene Bends, tends to remain 2. Polypropylene Unbends most of the way 3. Polystyrene Cracks but retains bend 4. ABS Bend tends to remain 5. PVC (Rigid) Bends easily and springs back quickly 6. Cellulose acetate Bend tends to remain 7. PMMA Cracks and splinters 8. Nylon Difficult to bend, springs back 9. Polycarbonate Tough to bend Corporate Training & Planning 8

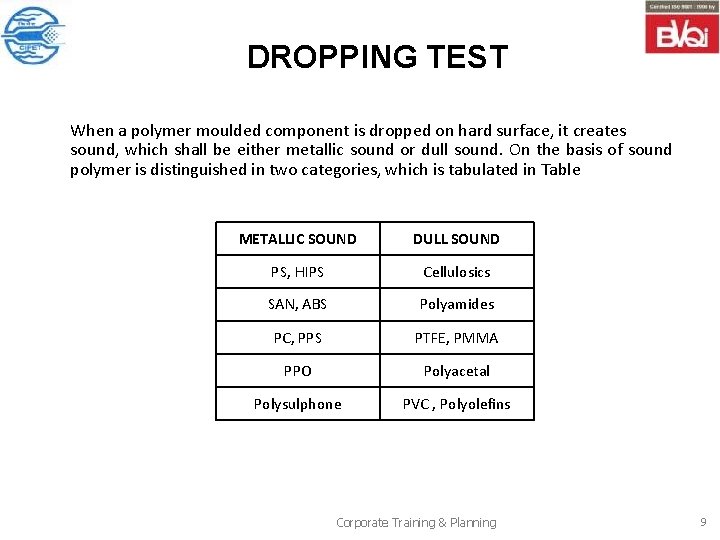
DROPPING TEST When a polymer moulded component is dropped on hard surface, it creates sound, which shall be either metallic sound or dull sound. On the basis of sound polymer is distinguished in two categories, which is tabulated in Table METALLIC SOUND DULL SOUND PS, HIPS Cellulosics SAN, ABS Polyamides PC, PPS PTFE, PMMA PPO Polyacetal Polysulphone PVC , Polyolefins Corporate Training & Planning 9

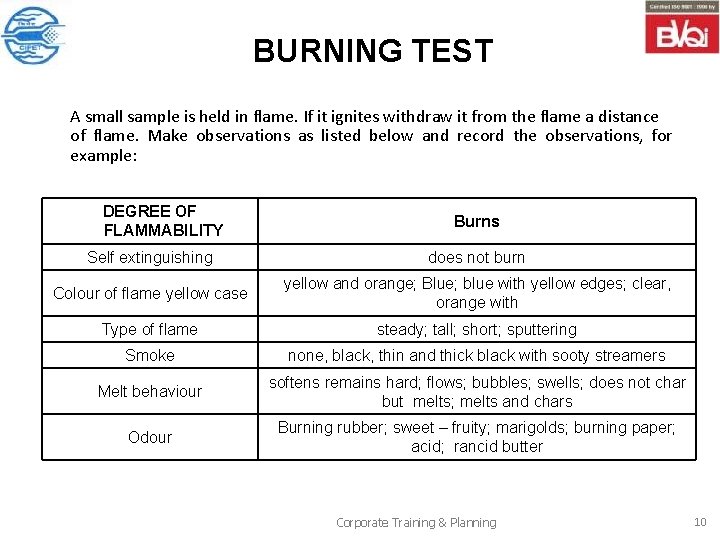
BURNING TEST A small sample is held in flame. If it ignites withdraw it from the flame a distance of flame. Make observations as listed below and record the observations, for example: DEGREE OF FLAMMABILITY Burns Self extinguishing does not burn Colour of flame yellow case yellow and orange; Blue; blue with yellow edges; clear, orange with Type of flame steady; tall; short; sputtering Smoke none, black, thin and thick black with sooty streamers Melt behaviour softens remains hard; flows; bubbles; swells; does not char but melts; melts and chars Odour Burning rubber; sweet – fruity; marigolds; burning paper; acid; rancid butter Corporate Training & Planning 10

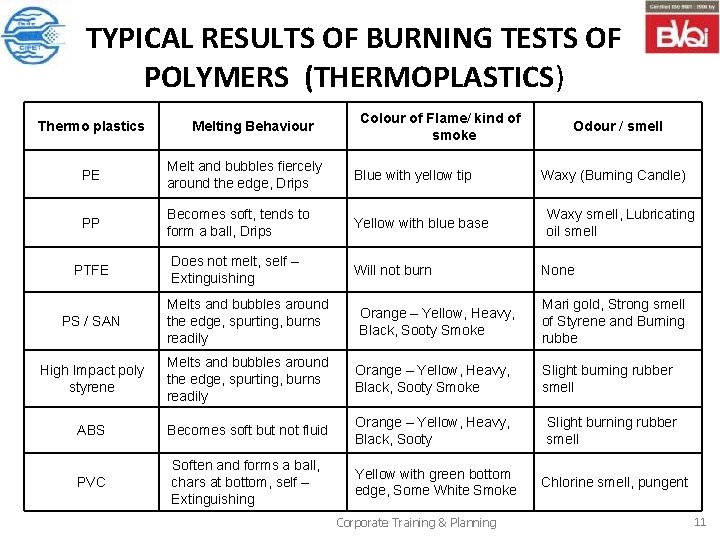
TYPICAL RESULTS OF BURNING TESTS OF POLYMERS (THERMOPLASTICS) Thermo plastics Melting Behaviour Colour of Flame/ kind of smoke PE Melt and bubbles fiercely around the edge, Drips Blue with yellow tip PP Becomes soft, tends to form a ball, Drips Yellow with blue base PTFE Does not melt, self – Extinguishing Will not burn Odour / smell Waxy (Burning Candle) Waxy smell, Lubricating oil smell None PS / SAN Melts and bubbles around the edge, spurting, burns readily High Impact poly styrene Melts and bubbles around the edge, spurting, burns readily Orange – Yellow, Heavy, Black, Sooty Smoke ABS Becomes soft but not fluid Orange – Yellow, Heavy, Black, Sooty Slight burning rubber smell PVC Soften and forms a ball, chars at bottom, self – Extinguishing Yellow with green bottom edge, Some White Smoke Chlorine smell, pungent Orange – Yellow, Heavy, Black, Sooty Smoke Corporate Training & Planning Mari gold, Strong smell of Styrene and Burning rubbe Slight burning rubber smell 11

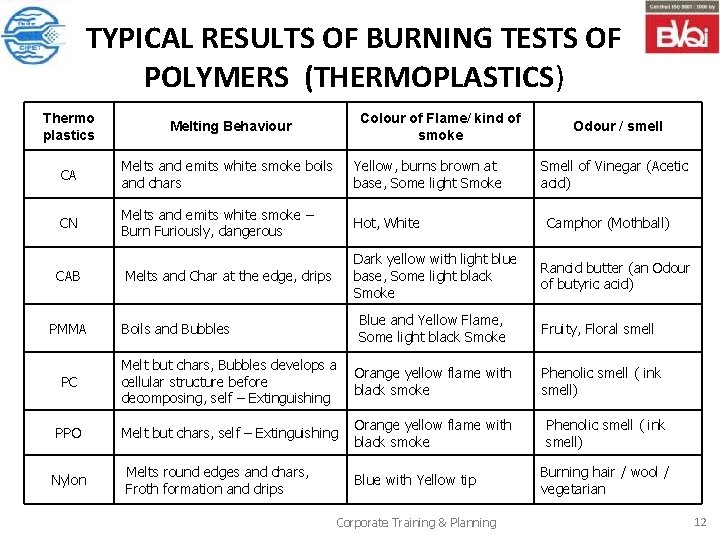
TYPICAL RESULTS OF BURNING TESTS OF POLYMERS (THERMOPLASTICS) Thermo plastics Melting Behaviour CA Melts and emits white smoke boils and chars Yellow, burns brown at base, Some light Smoke CN Melts and emits white smoke – Burn Furiously, dangerous Hot, White CAB Colour of Flame/ kind of smoke Dark yellow with light blue base, Some light black Smoke Melts and Char at the edge, drips Odour / smell Smell of Vinegar (Acetic acid) Camphor (Mothball) Rancid butter (an Odour of butyric acid) Blue and Yellow Flame, Some light black Smoke Fruity, Floral smell PC Melt but chars, Bubbles develops a cellular structure before decomposing, self – Extinguishing Orange yellow flame with black smoke Phenolic smell ( ink smell) PPO Melt but chars, self – Extinguishing Orange yellow flame with black smoke Melts round edges and chars, Froth formation and drips Blue with Yellow tip PMMA Nylon Boils and Bubbles Corporate Training & Planning Phenolic smell ( ink smell) Burning hair / wool / vegetarian 12

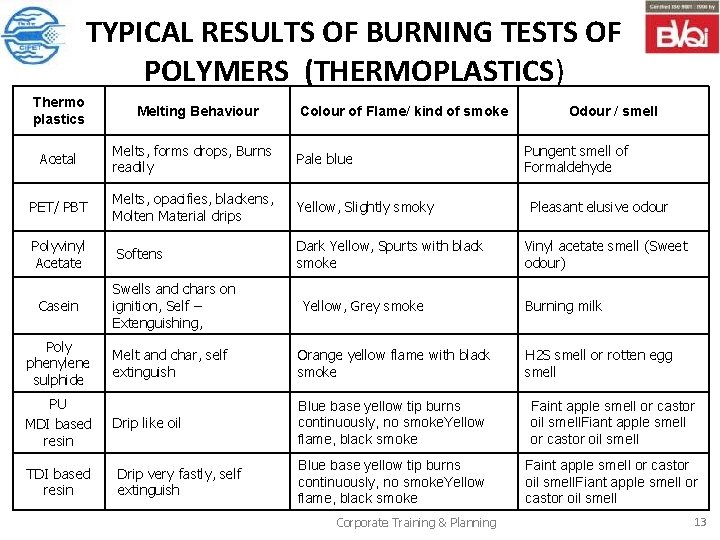
TYPICAL RESULTS OF BURNING TESTS OF POLYMERS (THERMOPLASTICS) Thermo plastics Melting Behaviour Colour of Flame/ kind of smoke Acetal Melts, forms drops, Burns readily Pale blue PET/ PBT Melts, opacifies, blackens, Molten Material drips Yellow, Slightly smoky Polyvinyl Acetate Softens Dark Yellow, Spurts with black smoke Odour / smell Pungent smell of Formaldehyde Pleasant elusive odour Vinyl acetate smell (Sweet odour) Casein Swells and chars on ignition, Self – Extenguishing, Poly phenylene sulphide Melt and char, self extinguish Orange yellow flame with black smoke PU MDI based resin Drip like oil Blue base yellow tip burns continuously, no smoke. Yellow flame, black smoke Faint apple smell or castor oil smell. Fiant apple smell or castor oil smell TDI based resin Drip very fastly, self extinguish Blue base yellow tip burns continuously, no smoke. Yellow flame, black smoke Faint apple smell or castor oil smell. Fiant apple smell or castor oil smell Yellow, Grey smoke Corporate Training & Planning Burning milk H 2 S smell or rotten egg smell 13

TYPICAL RESULTS OF BURNING TESTS OF POLYMERS (THERMOPLASTICS) THERMOSET COLOUR OF FLAME/ KIND OF SMOKE Phenol Resin Yellow flame, self extinguishing, Some sparks Charcoal smell Urea Resin Yellow with light blue edge, self extinguishing Fishy smell Melamine Resin Yellow with light blue edge, self extinguishing Fishy smell Burn with orange yellow flame, Black smoke Ester smell Yellow flame, Black smoke Charred flour smell Polyester Resin (Unsaturated) Epoxy Corporate Training & Planning ODOUR/ SMELL 14

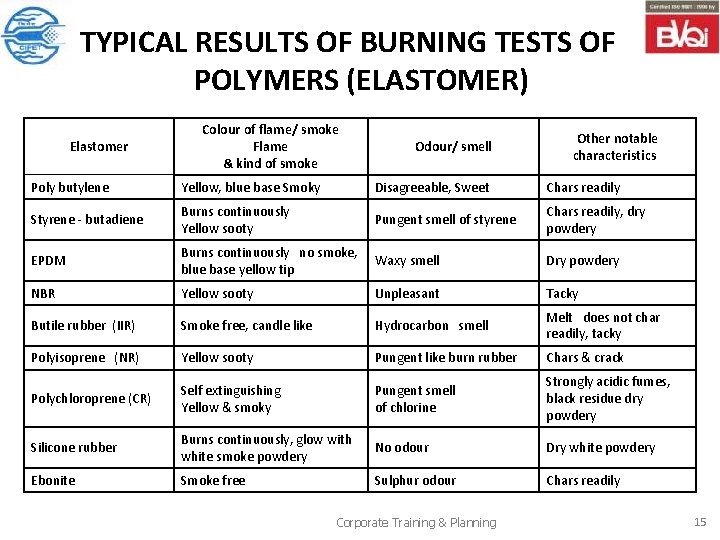
TYPICAL RESULTS OF BURNING TESTS OF POLYMERS (ELASTOMER) Elastomer Colour of flame/ smoke Flame & kind of smoke Odour/ smell Other notable characteristics Poly butylene Yellow, blue base Smoky Disagreeable, Sweet Chars readily Styrene - butadiene Burns continuously Yellow sooty Pungent smell of styrene Chars readily, dry powdery EPDM Burns continuously no smoke, blue base yellow tip Waxy smell Dry powdery NBR Yellow sooty Unpleasant Tacky Butile rubber (IIR) Smoke free, candle like Hydrocarbon smell Melt does not char readily, tacky Polyisoprene (NR) Yellow sooty Pungent like burn rubber Chars & crack Polychloroprene (CR) Self extinguishing Yellow & smoky Pungent smell of chlorine Strongly acidic fumes, black residue dry powdery Silicone rubber Burns continuously, glow with white smoke powdery No odour Dry white powdery Ebonite Smoke free Sulphur odour Chars readily Corporate Training & Planning 15

PYROLYSIS TESTS • In this testing, heat few milligrams of the sample in an ignition tube and test the pyrolytic vapour with a moistened indicator paper. The behaviour of vapour to indicator paper is given as follows: – ACID: turns blue litmus to red – BASE: turns red litmus to blue. – ACID VAPOURS: may come from carbohydrate polymers & their derivatives. [e. g. , cellulose acetate] – HIGH ACID VAPOURS: often indicates the presence of chlorine. e. g. , PVC or rubber neutral vapors] evolved from hydro carbon polymers, silicones and some polyesters hydrochloride. – ALKALINE VAPOURS: indicate the presence of N 2. e. g. polyamide, proteins & amino formaldehyde resins. Corporate Training & Planning 16

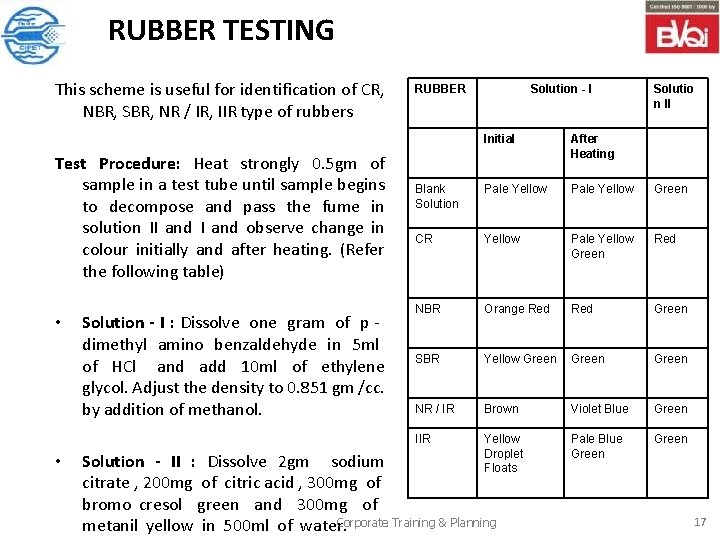
RUBBER TESTING This scheme is useful for identification of CR, NBR, SBR, NR / IR, IIR type of rubbers Test Procedure: Heat strongly 0. 5 gm of sample in a test tube until sample begins to decompose and pass the fume in solution II and observe change in colour initially and after heating. (Refer the following table) • • Solution - I : Dissolve one gram of p dimethyl amino benzaldehyde in 5 ml of HCl and add 10 ml of ethylene glycol. Adjust the density to 0. 851 gm /cc. by addition of methanol. RUBBER Solution - I Solutio n II Initial After Heating Blank Solution Pale Yellow Green CR Yellow Pale Yellow Green Red NBR Orange Red Green SBR Yellow Green NR / IR Brown Violet Blue Green IIR Yellow Droplet Floats Pale Blue Green Solution - II : Dissolve 2 gm sodium citrate , 200 mg of citric acid , 300 mg of bromo cresol green and 300 mg of Corporate Training & Planning metanil yellow in 500 ml of water. 17

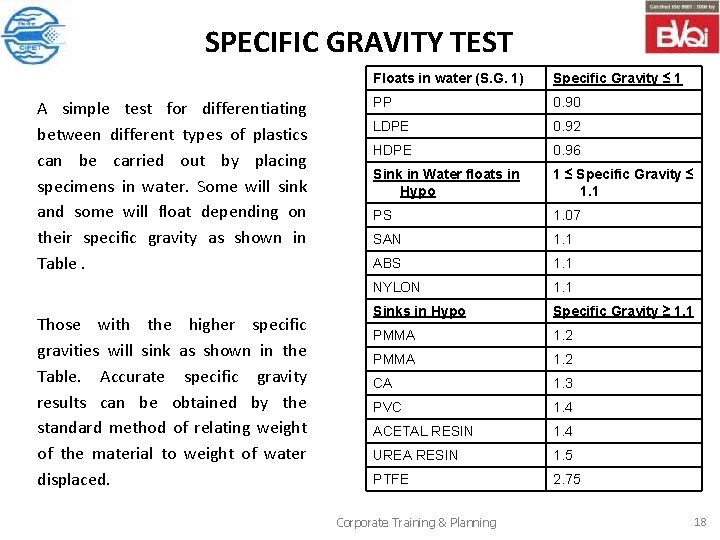
SPECIFIC GRAVITY TEST A simple test for differentiating between different types of plastics can be carried out by placing specimens in water. Some will sink and some will float depending on their specific gravity as shown in Table. Those with the higher specific gravities will sink as shown in the Table. Accurate specific gravity results can be obtained by the standard method of relating weight of the material to weight of water displaced. Floats in water (S. G. 1) Specific Gravity ≤ 1 PP 0. 90 LDPE 0. 92 HDPE 0. 96 Sink in Water floats in Hypo 1 ≤ Specific Gravity ≤ 1. 1 PS 1. 07 SAN 1. 1 ABS 1. 1 NYLON 1. 1 Sinks in Hypo Specific Gravity ≥ 1. 1 PMMA 1. 2 CA 1. 3 PVC 1. 4 ACETAL RESIN 1. 4 UREA RESIN 1. 5 PTFE 2. 75 Corporate Training & Planning 18

SOLUBILITY TESTS Solubility tests form a basis of some older identification schemes for main types of plastic materials. However, in many cases solubility varies considerably for different samples of the same resin and it is difficult to interpret the results Solubility of plastics may vary according to the grade or to the whether or not other constituents are present in the sample. The data in Table refer in principle to pure polymers, although even those polymers may exhibit differences in solubility. The solubility test should be carried out directly in a test tube. To about 100 mg of a powdered sample add 10 ml of solvent, mix occasionally shake the contents of the test tube and observe for a few hours swelling may occur before complete dissolution of the polymer. Corporate Training & Planning 19

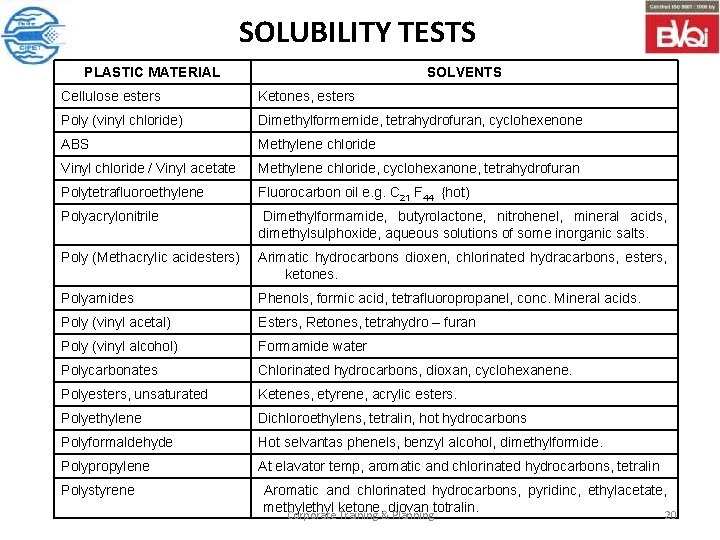
SOLUBILITY TESTS PLASTIC MATERIAL SOLVENTS Cellulose esters Ketones, esters Poly (vinyl chloride) Dimethylformemide, tetrahydrofuran, cyclohexenone ABS Methylene chloride Vinyl chloride / Vinyl acetate Methylene chloride, cyclohexanone, tetrahydrofuran Polytetrafluoroethylene Fluorocarbon oil e. g. C 21 F 44 {hot) Polyacrylonitrile Dimethylformamide, butyrolactone, nitrohenel, mineral acids, dimethylsulphoxide, aqueous solutions of some inorganic salts. Poly (Methacrylic acidesters) Arimatic hydrocarbons dioxen, chlorinated hydracarbons, esters, ketones. Polyamides Phenols, formic acid, tetrafluoropropanel, conc. Mineral acids. Poly (vinyl acetal) Esters, Retones, tetrahydro – furan Poly (vinyl alcohol) Formamide water Polycarbonates Chlorinated hydrocarbons, dioxan, cyclohexanene. Polyesters, unsaturated Ketenes, etyrene, acrylic esters. Polyethylene Dichloroethylens, tetralin, hot hydrocarbons Polyformaldehyde Hot selvantas phenels, benzyl alcohol, dimethylformide. Polypropylene At elavator temp, aromatic and chlorinated hydrocarbons, tetralin Polystyrene Aromatic and chlorinated hydrocarbons, pyridinc, ethylacetate, methyl ketone, diovan totralin. Corporate Training & Planning 20

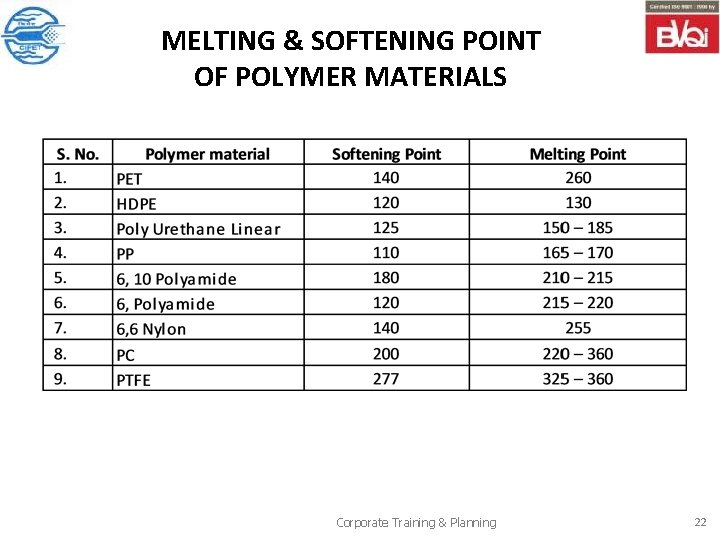
SOFTENING AND MELTING POINTS The temperature range of softening and melting – point can be used to characterize/ identify the material. Procedure: Place the 2 to 3 mg portion on a clean glass slide and cover with No 1 cover glass. Heat the slide, sample and cover on a hot plate to slightly above the softening point of the polymer so that a thin film can be formed. By slightly pressor on the cover glass form a thin film 0. 01 to 0. 04 mm, and allow it to cool slowly by turning of the hot plate power to promote crystallization. Keep the slide on hot plate melting point apparatus. Adjust the hot plate temperature about 10 0 C below the melting point of sample. Then adjust the temperature ramp at 0. 5 0 C/ min. observe the sample through microscope and note down the temperature where the material starts melting. Corporate Training & Planning 21

MELTING & SOFTENING POINT OF POLYMER MATERIALS Corporate Training & Planning 22

ELEMENTAL ANALYSIS • The results of test for the elements other than carbon, hydrogen and oxygen, namely nitrogen, sulphur, and halogens serve to indicate the possible nature of the unknown material • It should be noted that the compounding ingredients may contain elements which will also be detected and thus give rise to a positive result. They may interfere with the identification of the polymer. So additives free extracted purified polymer should be used for elemental analysis in order to identify exactly the nature of polymer. Corporate Training & Planning 23

CARBON Experiment: 0. 05 gm of material is mixed with 0. 2 gm of potassium dichromate, 10 drops of phosphoric acid in a test tube. Exclude air present by blowing with CO 2 with N 2 or O 2. Heat the solution in H 2 SO 4 or glycerol bath at 200 o. C. Connect test tube to a U – tube containing clear Ba. Cl 2 solution. Observation: White precipitate of Ba. Co 3 Inference: Carbon confirmed Corporate Training & Planning 24

HYDROGEN Experiment: Place little of the sample in a micro test tube with few o-g- of pure sulphur cover mouth strain with led-acetate paper. Heat the solution in glycerol bath for 2 min. Observation: Brown black strain Inference: Hydrogen Confirmed Corporate Training & Planning 25

OXYGEN Experiment: Reagent preparation: Dissolve 1 gm of ferric chloride and 1 gm of potassium thiocyanate separately in 10 ml of methanol. Mix the two solutions and after standing for few hours filter of the KCl precipitate. Dip strips of filter paper in methanol solution. Dry it is air, Prepare fresh strips before test. Several drops of liquid polymer or its solution are placed on the paper prepared above (conduct a blank also) Observation: Deep wine red colour Inference: Oxygen confirmed Corporate Training & Planning 26

NITROGEN Experiment: 2 ml of the sodium fusion extract is boiled with 3 drops of freshly prepared aqueous ferrous sulphate (approx 5%) & then cooled, after acidification with acid, a drop of 0. 5 N aqueous ferric chloride is added Observation: Blue precipitate Inference: Nitrogen confirmed Corporate Training & Planning 27

SULPHUR Experiment: Three drops of freshly prepared aqueous sodium nitroprusside (approx 5%) are added to the test portion. Observation: Violet colour Inference: Sulphur confirmed Corporate Training & Planning 28

CHLORINE & BROMINE Experiment: The test portion is acidified with 5 N Nitric acid; the solution is boiled for 2 min. 0. 1 N aqueous silver nitrate is then added. Observation & Inference: A white precipitate which is soluble in ammonia, Chlorine confirmed. A yellow precipitate which is insoluble in ammonia, Bromine confirmed. Corporate Training & Planning 29

FLUORINE Experiment: • An aqueous solution of Zirconium nitrate (0. 1%) and alizarin reds (0. 1%) is prepared. • Filter paper is immersed in the solution and then allowed to dry. When required, a small piece of the paper is moisturened with aqueous acetic acid (50%). • The solution to be tested is neutralized with 5 N HCL and a drop placed on the moistened test paper. Observation: Red spot turns Yellow Inference: Fluorine Confirmed. Corporate Training & Planning 30

HALOGEN: COPPER WIRE TEST • Take the piece of copper wire about 5 mm long. Push on end of the wire in to a small cork. (The cork is used as a handle so you are not touching a hot wire. • Place one pellet or plastics sample near your Bunsen burner. This is the sample you will be testing. • Hold the free end of the copper wire in the burner flame until it is red-hot and the flame no longer has a green colour. • Remove the wire from the flame and torch the hot wire to the plastics pellet or sample you will be testing. A small amount of the plastics should melt onto the wire. If the wire sticks to the plastic sample, use a pair of tongs to remove it. • Place the end of the wire, with small amount of plastic on it, into the flame. You should see a slight flash of a luminous flame (a yellow- orange colour). If the flame turns green in colour, then the sample contains chlorine. Corporate Training & Planning 31

CONFIRMATION TEST - THERMOPLASTICS Tests for Polyolefins A piece of dry sample is pyrolysed in a tube closed with a filter paper which is drenched with a solution of 0. 5 g yellow mercury (II) oxide in sulphuric acid (1. 5 ml conc. Sulphuric acid added to 8 ml. Water). If the vapour gives a golden yellow spot, indicates polyisobutylene, butyl rubber and polypropylene (the latter only after a few minutes). Polyethylene does not react. Natural and nitrile rubber, as well as polybutadiene yield a brown spot. Corporate Training & Planning 32

CONFIRMATION TEST - THERMOPLASTICS Cont. Test for Chlorine containing polymers • Heat a small amount of polymer in pyridine and make a solution. • Add few drops of 5% alcoholic (Methanol) Na. OH to a portion of boiling solution and a portion of solution in cool condition. • Observe the change of colour taking place in the solutions. • Table 6 is the list of colours observed in various chlorine containing polymers. Corporate Training & Planning 33

CONFIRMATION TEST - THERMOPLASTICS Cont. Test for Caprolactum in Nylon 6 • About 0. 5 gm of sample is heated in 5. 0 ml of distilled water and allowed to boil for 10 to 15 min. • After cooling, 2 -3 drops of con. H 2 SO 4 is added to 0. 5 ml of the above solution followed by addition of 2 ml. of potassium iodo bismuthate (a solution of 5 gm of basic bismuth nitrate and 25 gm. of potassium iodide in 10 ml. of 2% H 2 SO 4 ). • Precipitation of an orange red complex indicates the presence of caprolactum. Corporate Training & Planning 34

CONFIRMATION TEST - THERMOPLASTICS Cont. Test for Adipic acid in Nylon 6, 6 • About 0. 2 gm of sample is heated in low flame in a test tube and the vapour coming out of the tube is passed over a filter paper moistened with a saturated solution of O-nitrobenzaldehyde in 2 N aqueous Na. OH. • A deep mauve (violet) colour confirms the presence of adipic acid. • A yellowish green colour develops in the case of polyethylenete rephthalate and polybutyleneterephthalate confirming the presence of terephthalic acid. Corporate Training & Planning 35

CONFIRMATION TEST - THERMOPLASTICS Cont. Test for Polycarbonate • About 1. 0 gm of sample is pyrolysed in an ignition tube, which is plugged with cotton. • The cotton is removed and immersed in 1% methanolic solution of p-dimethylamino benzaldehyde and then one drop of 5 N-Hydrochloric acid is added. • A dark blue colour appears in the case of polycarbonates. • A red colour that does not change to blue is observed in the case of polyamides. • The cotton floak in which the prolysed vapours of the sample absorbed is treated with dilute (1: 1) HCL. • An intense red colour which is unaffected by methanol indicates the presence of polycarbonate. Corporate Training & Planning 36

CONFIRMATION TEST - THERMOPLASTICS Cont. Test for PMMA • About 1 gm of sample is heated in an ignition tube and the pyrolysate is collected in a test tube wrapped with a wet filter paper. • To the distillate, 1 ml. of conc. HNO 3 is added and heated just to boiling. • After cooling, 5 ml. of water is added an then sodium nitrite (0. 1 gm) is added. • A blueish green colour confirms the presence of polymethylmethacrylate. Corporate Training & Planning 37

CONFIRMATION TEST - THERMOPLASTICS Cont. Test for Polyacetals • Polyacetals produce formaldehyde on heating. • A small amount of sample is heated with 2 ml. conc. Sulphuric acid and a few crystals of chromotropic acid for about 10 min at 60 -70 C. • A strong violet colour indicates formaldehyde. Corporate Training & Planning 38

CONFIRMATION TEST - THERMOPLASTICS Cont. Tests for PET and PBT • PET and PBT are soluble in nitrobenzene. • A small sample is pyrolysed in a glass tube covered with filter paper. • The filter paper is drenched with a saturated solution of O-nitroben zaldehyde in dilute sodium hydroxide. • A blue-green colour, which is stable against dilute hydrochloric acid, indicates terephthalic acid. • Then PET and PBT are differentiated based on melting points. Corporate Training & Planning 39

CONFIRMATION TEST - THERMOPLASTICS Cont. Test for polyurethane • About 0. 5 gm of sample is dissolved in 10 ml of glacial acetic acid and 0. 1 gm of p-dimethylamino benzaldehyde is added. • The solution turning yellow after several minutes shows the presence of polyurethane. Corporate Training & Planning 40

CONFIRMATION TEST - THERMOPLASTICS Cont. Test for polyurethane • About 0. 5 gm of sample is dissolved in 10 ml of glacial acetic acid and 0. 1 gm of p-dimethylamino benzaldehyde is added. • The solution turning yellow after several minutes shows the presence of polyurethane. Corporate Training & Planning 41

CONFIRMATION TEST - THERMOPLASTICS Cont. Test for Cellulose in Cellulosics (Molisch Reaction) Sample is dissolved in acetone, reacted with 2 to 3 drops of 2% ethanolic solution of 1 napthol and a few drops of conc. H 2 S 04. is allowed to flow down the wall of the test tube. Red to Reddish brown ring at the interface indicates cellulose. Corporate Training & Planning 42

CONFIRMATION TEST - THERMOPLASTICS Test for acetates and propionates Cont. • The sample (about 0. 2 gm) and few ml of Hydrochloric acid are heated for 10 minutes and dilute ammonia is added to make alkaline. • One ml of this solution is reacted with 1": 2 drops of a 5% lanthanom nitrate solution and 1 drop of 0. 1 N Iodine solutions. • A fast colour change to blue confirms the presence of acetates and a brown colour indicates the presence of propionate. • For differentiation between cellulose acetate and cellulose acetate-butyrate, it is usually sufficient to examine the vapour produced by dry heating of the sample. • The acetate smells like acetic acid, the acetatebutryate has the smell of both acetic acid and butyric acid (like rancid butter). Corporate Training & Planning 43

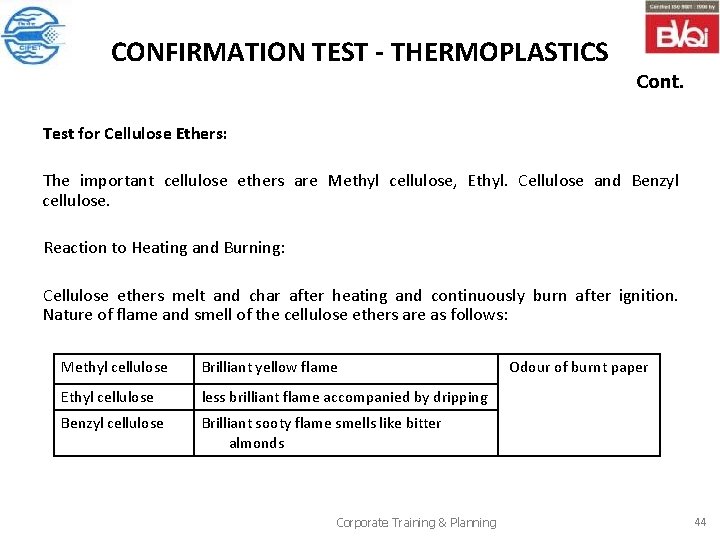
CONFIRMATION TEST - THERMOPLASTICS Cont. Test for Cellulose Ethers: The important cellulose ethers are Methyl cellulose, Ethyl. Cellulose and Benzyl cellulose. Reaction to Heating and Burning: Cellulose ethers melt and char after heating and continuously burn after ignition. Nature of flame and smell of the cellulose ethers are as follows: Methyl cellulose Brilliant yellow flame Ethyl cellulose less brilliant flame accompanied by dripping Benzyl cellulose Brilliant sooty flame smells like bitter almonds Corporate Training & Planning Odour of burnt paper 44

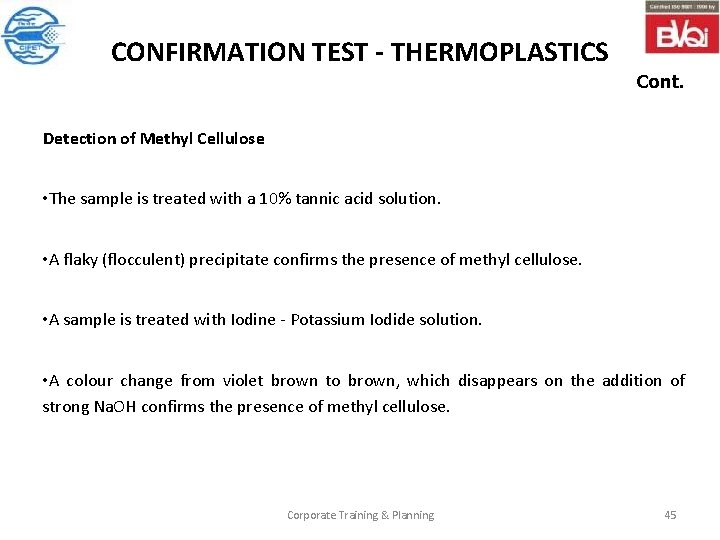
CONFIRMATION TEST - THERMOPLASTICS Cont. Detection of Methyl Cellulose • The sample is treated with a 10% tannic acid solution. • A flaky (flocculent) precipitate confirms the presence of methyl cellulose. • A sample is treated with Iodine - Potassium Iodide solution. • A colour change from violet brown to brown, which disappears on the addition of strong Na. OH confirms the presence of methyl cellulose. Corporate Training & Planning 45

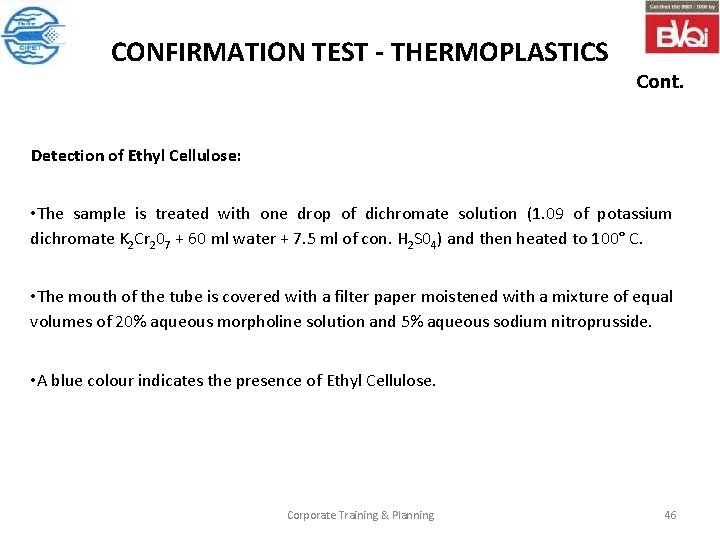
CONFIRMATION TEST - THERMOPLASTICS Cont. Detection of Ethyl Cellulose: • The sample is treated with one drop of dichromate solution (1. 09 of potassium dichromate K 2 Cr 207 + 60 ml water + 7. 5 ml of con. H 2 S 04) and then heated to 100° C. • The mouth of the tube is covered with a filter paper moistened with a mixture of equal volumes of 20% aqueous morpholine solution and 5% aqueous sodium nitroprusside. • A blue colour indicates the presence of Ethyl Cellulose. Corporate Training & Planning 46

CONFIRMATION TEST - THERMOPLASTICS Cont. Cellulose Nitrate • 1 ml of water is added to a test tube containing about 1 mg of the sample & 2 ml of freshly prepared 0. 2% solution of enthrone in conc. H 2 S 04. A green colour changing to dark blue is confirmed cellulose. • 20 ml of diphenylamine is dissolved in 1 ml of conc H 2 S 04. Few drops of this are poured on to a small piece of the sample on a spotting plate. Immediate development of intense blue colour is confirmed Nitrate. • Wash the sample with hot water dissolve in conc. H 2 S 04. Add a small amount of resorcinol. A purple blue colour development is confirmed Nitrate. Corporate Training & Planning 47

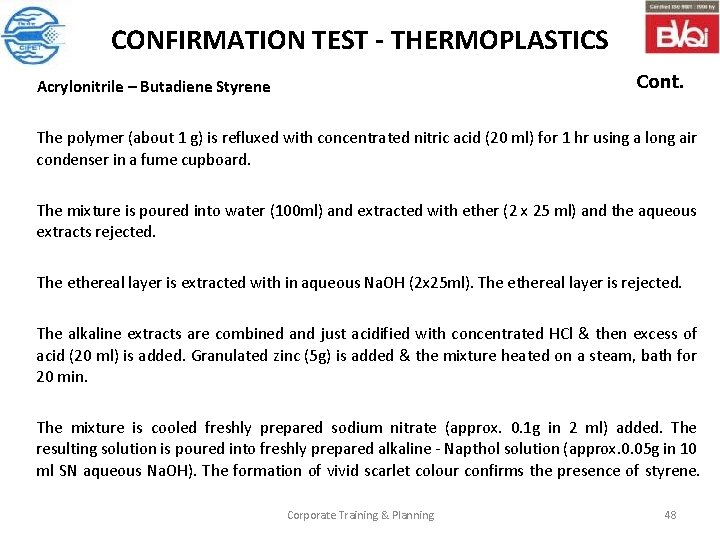
CONFIRMATION TEST - THERMOPLASTICS Cont. Acrylonitrile – Butadiene Styrene The polymer (about 1 g) is refluxed with concentrated nitric acid (20 ml) for 1 hr using a long air condenser in a fume cupboard. The mixture is poured into water (100 ml) and extracted with ether (2 x 25 ml) and the aqueous extracts rejected. The ethereal layer is extracted with in aqueous Na. OH (2 x 25 ml). The ethereal layer is rejected. The alkaline extracts are combined and just acidified with concentrated HCl & then excess of acid (20 ml) is added. Granulated zinc (5 g) is added & the mixture heated on a steam, bath for 20 min. The mixture is cooled freshly prepared sodium nitrate (approx. 0. 1 g in 2 ml) added. The resulting solution is poured into freshly prepared alkaline - Napthol solution (approx. 0. 05 g in 10 ml SN aqueous Na. OH). The formation of vivid scarlet colour confirms the presence of styrene. Corporate Training & Planning 48

CONFIRMATION TEST - THERMOPLASTICS Cont. Casein • A sample about (0. 02 g) is dissolved in concentrated HNO 3 (2 ml) by boiling for about 5 mn. • The solution is cooled and excess 5 N ammonium hydroxide added. • The formation of orange colour confirms the presence of casein. Corporate Training & Planning 49

CONFIRMATION TEST - THERMOPLASTICS Cont. Polyisobutylene • Filter paper is immersed in freshly prepared mercuric sulphate solution (prepared by dissolving yellow mercuric oxide (1 g) in boiling 5 N H 2 SO 4 (20 ml) and cooling before use and used without drying. • The paper is inserted in the mouth of an ignition tube containing the polymer (about 0. 2 g). The tube is gently heated. The formation of Bright yellow colour confirms the presence of Polyisobutylene. Corporate Training & Planning 50

CONFIRMATION TEST - THERMOPLASTICS Cont. Polymethyl Methacrylate • A sample (about 0. 1) is heated in an ignition tube and the pyrolysate collected in a test tube wrapped in a wet filter paper. • To the distillate is added conc. HNO 3 ( 1 ml) and the mixture is heated just to boiling and then cooled water (5 ml) and then Sodium nitrite ( 0. 1 g) are added. • The formation of Blue colour confirms the presence of PMMA. Corporate Training & Planning 51

CONFIRMATION TEST - THERMOPLASTICS Cont. Polyvinyl Acetate An iodine reagent is prepared by dissolving iodine (0. 1 g) & potassiumiodide (1 g) in a mixture of water (10 ml) & ethanol (10 ml) and making upto 100 ml with 2 N HCL. The polymer (about 0. 05 g) is covered with the iodine reagent (1 ml). The effect is enhanced if water (10 ml) is added. The formation of Stained deep red colour confirms the presence of Polyvinylacetate. Corporate Training & Planning 52

CONFIRMATION TEST - THERMOPLASTICS Cont. Polyvinyl Alcohol • A Sample (about 0. 02 g) is dissolved in water 5 ml and iodine reagent described above (5 drops) added. • The formation of Blue colour confirms the presence of Polyvinyl alcohol. Corporate Training & Planning 53

CONFIRMATION TEST - THERMOPLASTICS Cont. Shellac • A sample (0. 05 g) is dissolved in ethanol (1 ml) with warming. • To the cooled solution is added water (about 1 ml) to give an emulsion like precipitate. 5 N aqueous Na. OH 2 drops is added to the mixture. • The colour change is reversed on acidification with 5 N HCL. • The formation of Violet - red colour confirms the presence of Shellac. Corporate Training & Planning 54

CONFIRMATION TEST - THERMOSET PLASTICS Test formaldehyde • A small amount of sample is boiled in water in presence of H 2 S 04 and filtered. Few drops of 5% aqueous chromotropic acid solution (1, 8 -dihydroxynaphthalene-3, 6 disulphonic acid) and excess of conc. H 2 S 04 are added to the filterate and heated at 1000 C for few minutes. • In the presence of formaldehyde, the solution turns violet/dark violet. Poly (vinyl formal), polyoxymethylenes, PF, UF and MF resins contain formaldehyde. Corporate Training & Planning 55

CONFIRMATION TEST - THERMOSET PLASTICS Cont. Test for Phenol In PF Million's Reagent: • About 10 gm of Hg is dissolved in 10 ml. of HN 03 by gentle heating and then diluted with 15 ml. of distilled water. • The sample is heated to boiling for two minutes with 1 ml. of clear million's reagent. Red colour indicates the presence of phenol in the sample. Corporate Training & Planning 56

CONFIRMATION TEST - THERMOSET PLASTICS Cont. Test for Urea in UF • About 0. 5 gm. of sample is refluxed for 30 min. with 20% acetic acid (50 m. I. ). • The mixture is cooled and filtered. To the filterate added a solution of (2 ml. ) xanthydrol in methanol (1% solution) and boiled for 1 -2 minutes. • White bulky precipitate confirms the presence of urea. Corporate Training & Planning 57

CONFIRMATION TEST - THERMOSET PLASTICS Cont. Test for Melamine in MF • About 0. 5 gm of sample is refluxed with 80% acetic acid (25 ml. ) for 30 min. , cooled and filtered. • Then the filterate is evaporated to dryness and added 2 ml. of water, cooled and filtered. • To the filtrate one drop of saturated aqueous picric acid is added. Yellow precipitate indicates the presence of melamine. Corporate Training & Planning 58

CONFIRMATION TEST - THERMOSET PLASTICS Cont. Tests for Epoxy Resin Foucry Test: • About 0. 25 gm. of sample is dissolved in 98% H 2 S 04 by slight warming. • After cooling 1 ml. of 63% HN 03 is added. • After shaking, the mixture is poured into 100 ml. of 5% aqueous Na. OH. • A bright red or orange red colour indicates the presence of epoxy resin. Corporate Training & Planning 59

CONFIRMATION TEST - THERMOSET PLASTICS Cont. Tests for Alkyd Resins • Oil modified polyesters are called as alkyd resins. • Since in most of the Alkyd resins, phthalic anhydride is used as the major polybasic constituent the presence of phthalic anhydride is identified by the following test. Corporate Training & Planning 60

CONFIRMATION TEST - THERMOSET PLASTICS Cont. Test for Phthalate The sample (0. 1 gm) is carefully melted in a test tube with 0. 3 gm. of crystalline ptlenol. , and one drop of concentrated sulphuric acid. After cooling, the melt is dissolved in water (10 -20 ml) and the solution is made alkaline with 5% Na. OH. A red colour indicates the pthalate which confirms the alkayd resins. Corporate Training & Planning 61

CONFIRMATION TEST - RUBBERS Chloroprene rubber (CR) • Shake 250 gm of sample with 2 ml of iodine solution. • If violet colour fades within 2 to 3 minutes. It indicates CR. • A persistent green flame with copper wire during burning indicates chlorine. Corporate Training & Planning 62

CONFIRMATION TEST - RUBBERS Cont. SBR- NR-IR If 2 ml of chloroform show appreciable darkening when shaken with 200 mg of sample of vulcanized rubber product, and repeat pyrolysis test. Corporate Training & Planning 63

CONFIRMATION TEST - RUBBERS Cont. NR- IR Extract a fresh portion of sample with acetone, and swell the extracted sample in a little chloroform and add few drop of bromine and wait for 2 min. add about 1 gm phenol and warm on a stream bath to remove chloroform a blue to red – violet colour indicate NR/IR. Corporate Training & Planning 64

CONFIRMATION TEST - RUBBERS SBR Cont. • Boil 2 gm of dried, acetone-extracted sample under reflux with 20 ml HNO 3 for 1 hr, and add 100 ml of water. • Extract with 50, 25 ml portions of diethyl ether. Combine the ether extracts and wash twice with 15 ml of water and extract with 15 ml portion of Na. OH solution and finally extract with 20 ml of water. • Discard the ether, combine the sodium hydroxide extract and water washing, make just acid with HCl and add 20 ml in excess. • Heat on a stream bath and reduce the nitro benzoic acid by adding 5 gm on granulated zinc. Make other solution alkaline with the Na. OH solution to just dissolve the precipitate. • Extract twice with ether and discard the ether. Make the aqueous solution acid with HCl, cool to room temperature and add 2 ml of Na. NO 2 solution. • Pour this diazotized solution into an excess of solution of B-Napthol in Na. OH. A vivid scarlet colour indicates SBR. Corporate Training & Planning 65

CONFIRMATION TEST - RUBBERS Cont. IIR Place about 1 gm of dried, acetone extracted sample in a tube and decompose it by heating and pass on the vapours to seconds test tube placed in ice, from these, the vapour are transferred to another tube contains 0. 5 gm of mercuric acetate in 15 ml of methanol, evaporate the methanol and boil the residue with 25 ml petroleum ether and get petroleum ether extract and evaporate petroleum ether to get mercuric derivate with melting point of 55 degree centigrade. Corporate Training & Planning 66

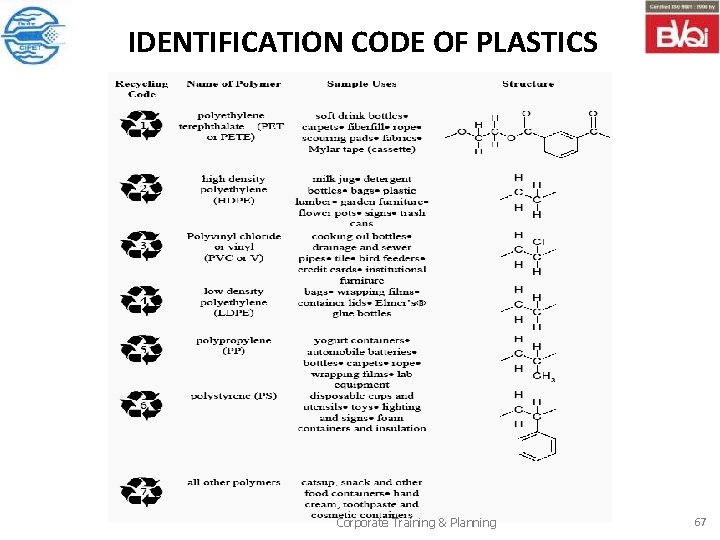
IDENTIFICATION CODE OF PLASTICS Corporate Training & Planning 67
