IDENTIFICATION OF IONS Testing For Halide Ions APPARATUS



















- Slides: 23

IDENTIFICATION OF IONS

Testing For Halide Ions APPARATUS

�TEST TUBES

�PIPETTE

�NITRIC ACID

�SILVER NITRATE

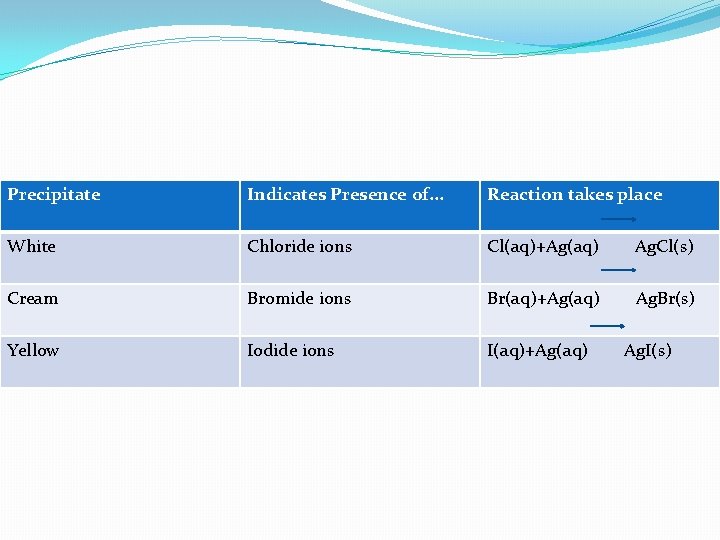
Method • • Take a small amount of the solution. Add an equal volume of dilute nitric acid. Then add silver nitrate solution. Silver halides are insoluble. So if Halide ions are present a precipitate will form, as shown in this table:

Precipitate Indicates Presence of. . . Reaction takes place White Chloride ions Cl(aq)+Ag(aq) Ag. Cl(s) Cream Bromide ions Br(aq)+Ag(aq) Ag. Br(s) Yellow Iodide ions I(aq)+Ag(aq) Ag. I(s)

Testing For Sulphate Ions APPARATUS

�TEST TUBES

�PIPETTE

�HYDROCLORIC ACID

�BARIUM NITRATE

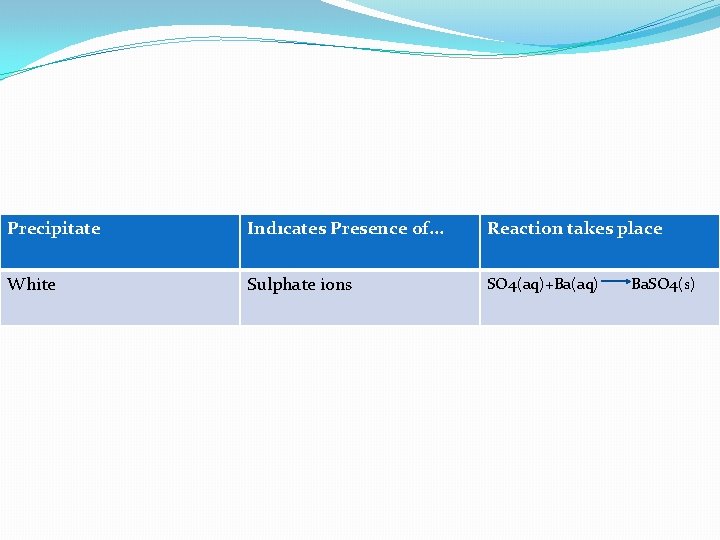
Method �Take a small amount of the solution. �Add an equal volume of dilute HCl. �Then add barium nitrate solution. �Barium sulphate is insoluble. So if sulphate ions are present a precipitate will form, as shown in this table.

Precipitate Indıcates Presence of. . . Reaction takes place White Sulphate ions SO 4(aq)+Ba(aq) Ba. SO 4(s)

Testing For Metal Ions APPARATUS

�TEST TUBES

�PIPETTE

�SODIUM HYDROXIDE

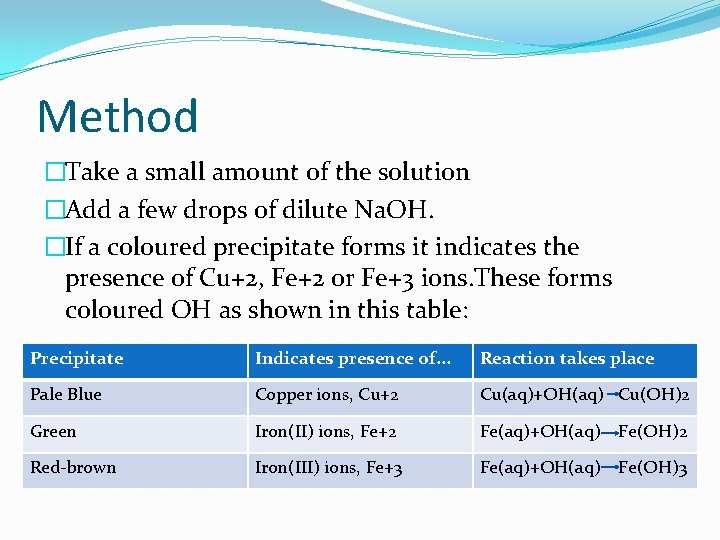
Method �Take a small amount of the solution �Add a few drops of dilute Na. OH. �If a coloured precipitate forms it indicates the presence of Cu+2, Fe+2 or Fe+3 ions. These forms coloured OH as shown in this table: Precipitate Indicates presence of. . . Reaction takes place Pale Blue Copper ions, Cu+2 Cu(aq)+OH(aq) Cu(OH)2 Green Iron(II) ions, Fe+2 Fe(aq)+OH(aq) Fe(OH)2 Red-brown Iron(III) ions, Fe+3 Fe(aq)+OH(aq) Fe(OH)3

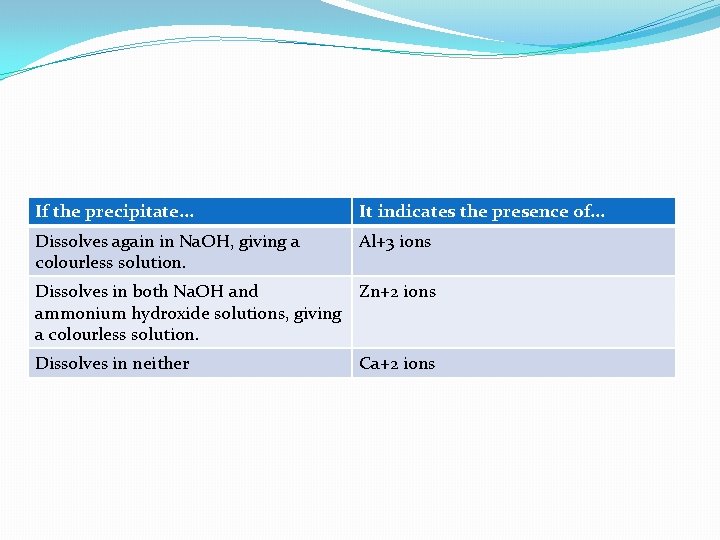
�If a white precipitate forms it suggest that Al+3, Zn+2 or Ca+2 ions are present. These form insoluble white OH. �To find out which of those three ions it is, divide the solution caontaining the precipitate into two equal volumes. �To one, add double the volume of Na. OH. To the other add double the volume of ammonium hydroxide solution. Check the reults against this table:

If the precipitate. . . It indicates the presence of. . . Dissolves again in Na. OH, giving a colourless solution. Al+3 ions Dissolves in both Na. OH and Zn+2 ions ammonium hydroxide solutions, giving a colourless solution. Dissolves in neither Ca+2 ions

REFERENCES � http: //images. asia. ru/img/alibaba/photo/51170673/Test_Tube. jpg � http: //www. ferret. com. au/odin/images/205834/Motorised-electronic-pipette-available-from-John. Morris-Scientific-205834. jpg � http: //www. amazingrust. com/Experiments/background_knowledge/Images/Nitric_Acid-big. jpg � http: //www. saltlakemetals. com/images/Silver. Nitrate. Bottle 50 g. png � http: //www. razor-gator. com/Skin. Acids. Photos/Bottle-HCl&Na. OH. jpg � http: //wpcontent. answers. com/wikipedia/commons/thumb/2/27/Dusičnan_barnatý. JPG/200 px. Dusičnan_barnatý. JPG � http: //genchem. wisc. edu/lab/CCA/STILLS 32 p. H/NAOHNACL. JPG