Ideal Gas Law and Daltons Law of Partial




- Slides: 9

Ideal Gas Law and Dalton’s Law of Partial Pressure

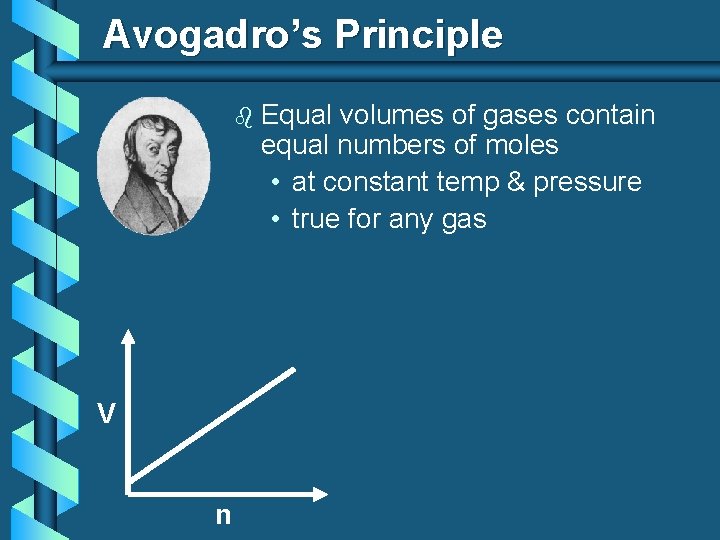
Avogadro’s Principle b V n Equal volumes of gases contain equal numbers of moles • at constant temp & pressure • true for any gas

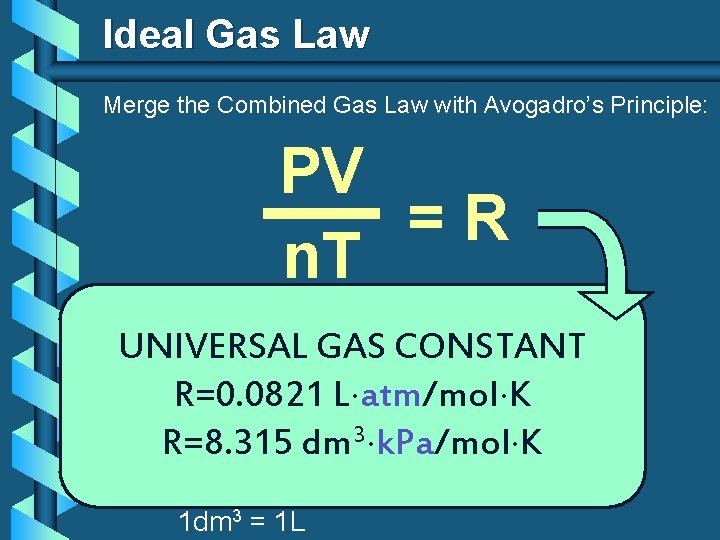
Ideal Gas Law Merge the Combined Gas Law with Avogadro’s Principle: PV V k =R n. T T n UNIVERSAL GAS CONSTANT R=0. 0821 L atm/mol K R=8. 315 dm 3 k. Pa/mol K 1 dm 3 = 1 L

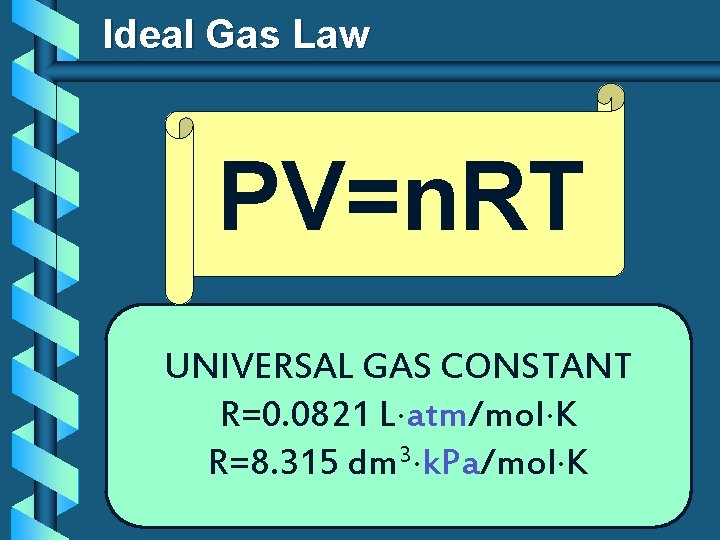
Ideal Gas Law PV=n. RT UNIVERSAL GAS CONSTANT R=0. 0821 L atm/mol K R=8. 315 dm 3 k. Pa/mol K

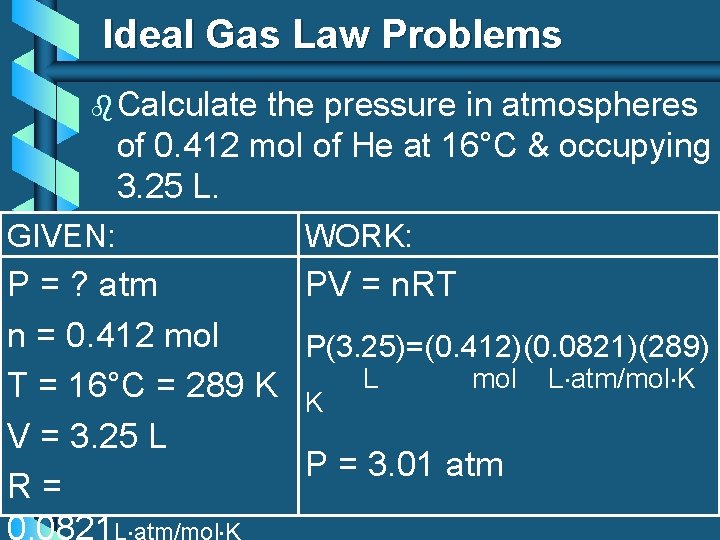
Ideal Gas Law Problems b Calculate the pressure in atmospheres of 0. 412 mol of He at 16°C & occupying 3. 25 L. GIVEN: WORK: P = ? atm PV = n. RT n = 0. 412 mol P(3. 25)=(0. 412)(0. 0821)(289) mol L atm/mol K T = 16°C = 289 K K L V = 3. 25 L P = 3. 01 atm R= 0. 0821 L atm/mol K

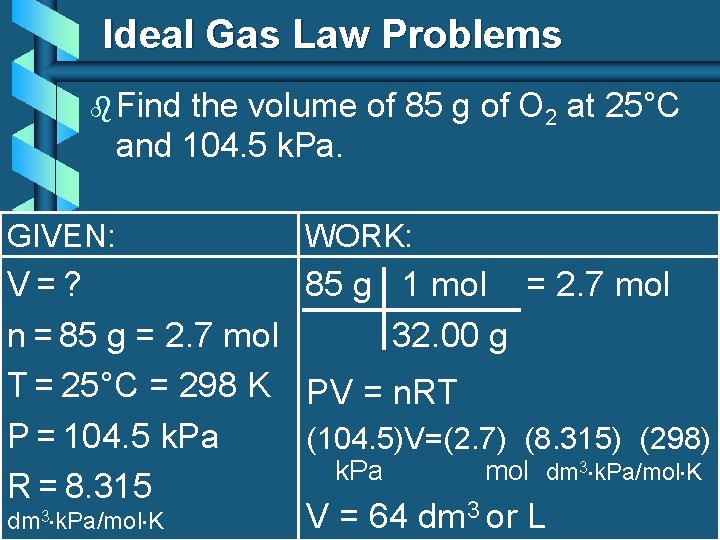
Ideal Gas Law Problems b Find the volume of 85 g of O 2 at 25°C and 104. 5 k. Pa. GIVEN: WORK: V=? 85 g 1 mol = 2. 7 mol n = 85 g = 2. 7 mol 32. 00 g T = 25°C = 298 K PV = n. RT P = 104. 5 k. Pa (104. 5)V=(2. 7) (8. 315) (298) k. Pa mol dm 3 k. Pa/mol K R = 8. 315 V = 64 dm 3 or L dm 3 k. Pa/mol K

Dalton’s Law of Partial Pressure The total pressure of a mixture of nonreacting gases is equal to the sum of the partial pressures of the individual gases (Ptotal = P 1 + P 2 + P 3 + …)

Problem 1 b 1 L of N 2 at 50 k. Pa is mixed with 1 L of O 2 at 60 k. Pa, to form a 1 L mixture of the gases. What is the resulting pressure? What are the partial pressures? b. Total pressure = 60 k. Pa + 50 k. Pa = 110 k. Pa

Problem 2 b b A balloon contains 75 k. Pa N 2, 15 k. Pa O 2, 5 k. Pa CO 2, and water vapour. If atmospheric pressure is 100 k. Pa what is the partial pressure of water vapour? 100 - (75 + 15 + 5) = 100 - 95 = 5 k. Pa

















