Ideal and Dilute Solutions Master Thermodynamics Equations Chemical


- Slides: 28

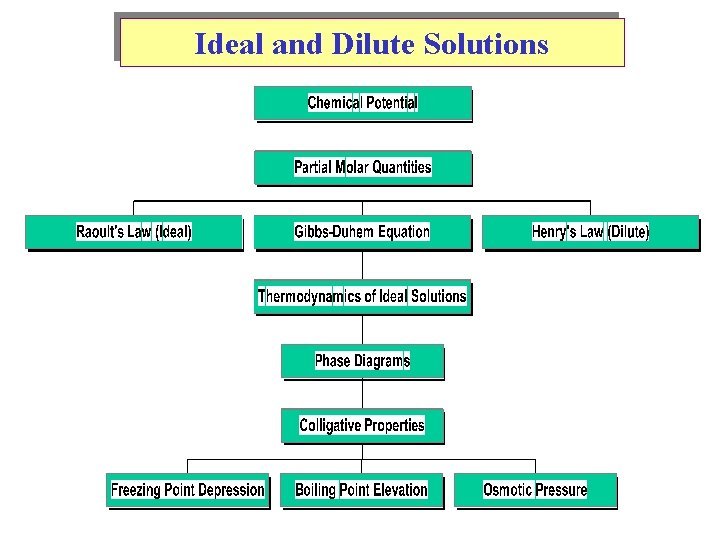
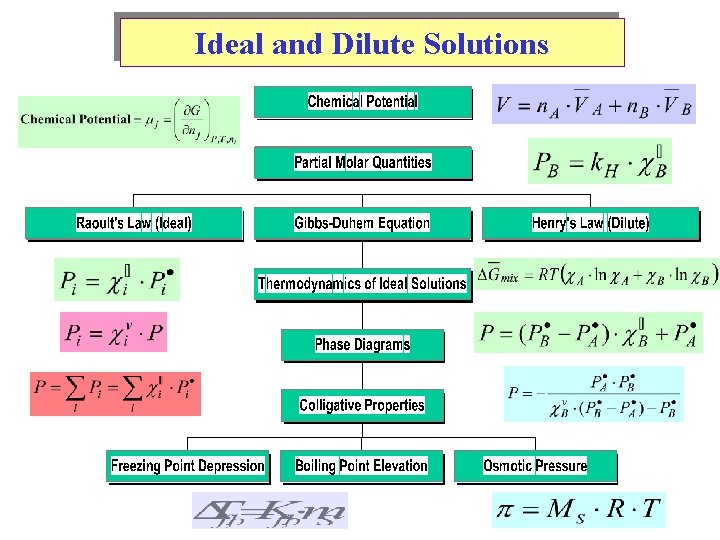
Ideal and Dilute Solutions

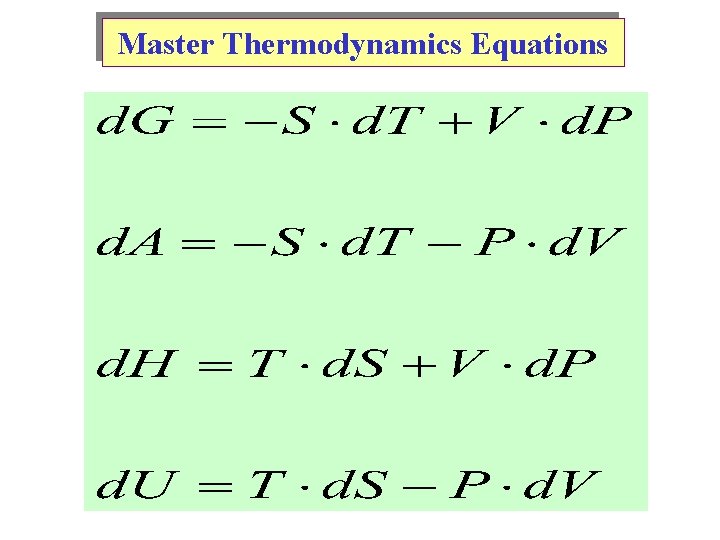
Master Thermodynamics Equations

Chemical Potential Diffusion from high to low potential. Chemical potential is a Partial Molar Quantity Sum of moles of components

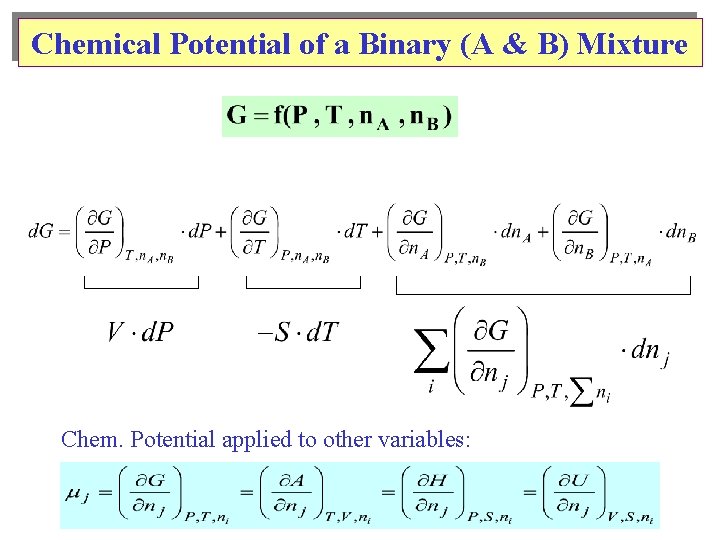
Chemical Potential of a Binary (A & B) Mixture Chem. Potential applied to other variables:

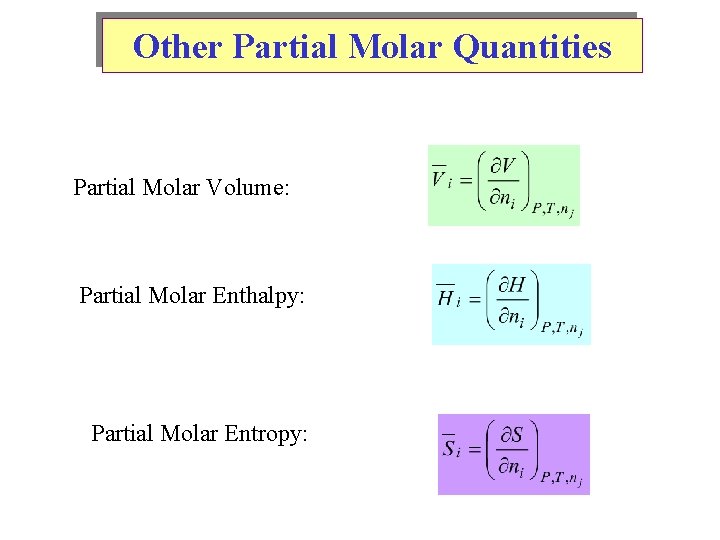
Other Partial Molar Quantities Partial Molar Volume: Partial Molar Enthalpy: Partial Molar Entropy:

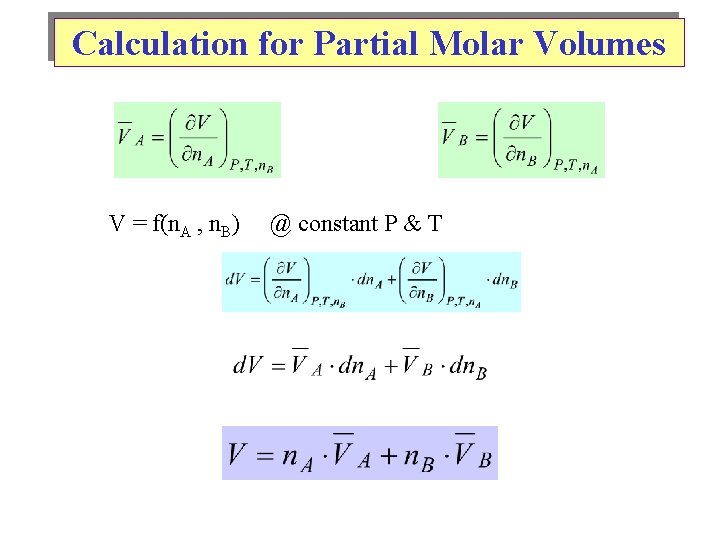
Calculation for Partial Molar Volumes V = f(n. A , n. B) @ constant P & T

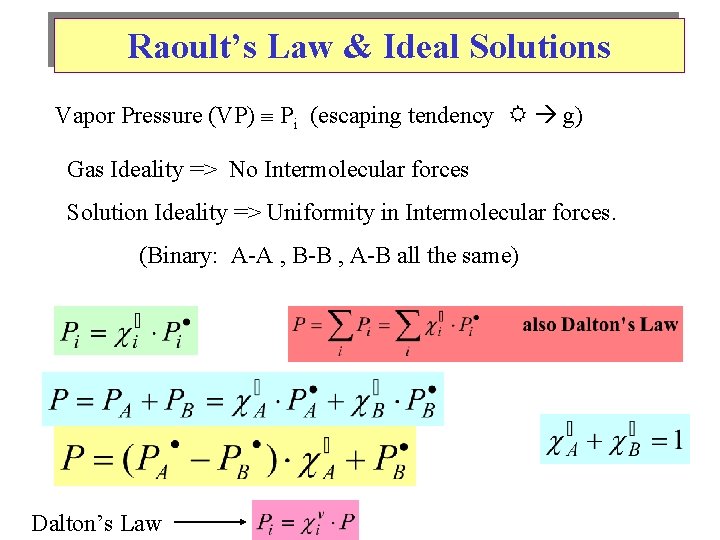
Raoult’s Law & Ideal Solutions Vapor Pressure (VP) Pi (escaping tendency g) Gas Ideality => No Intermolecular forces Solution Ideality => Uniformity in Intermolecular forces. (Binary: A-A , B-B , A-B all the same) Dalton’s Law

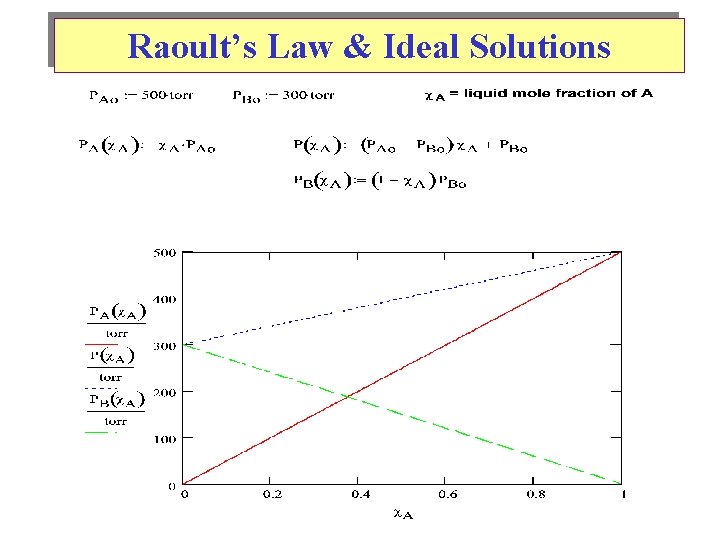
Raoult’s Law & Ideal Solutions

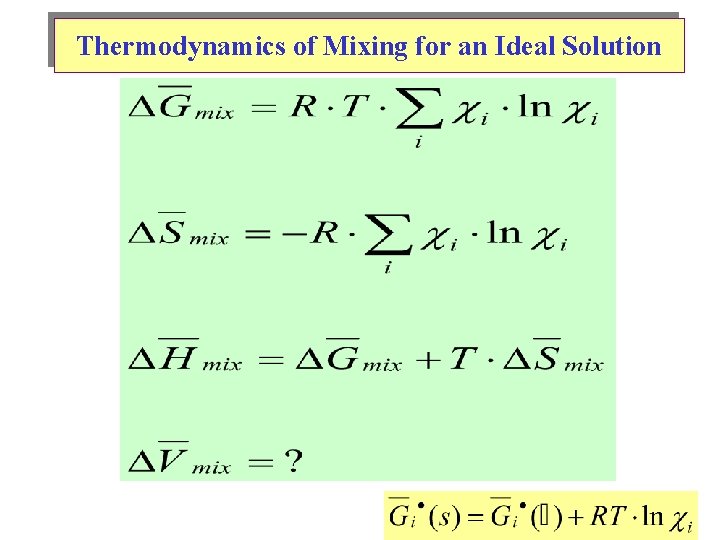
Thermodynamics of Mixing for an Ideal Solution

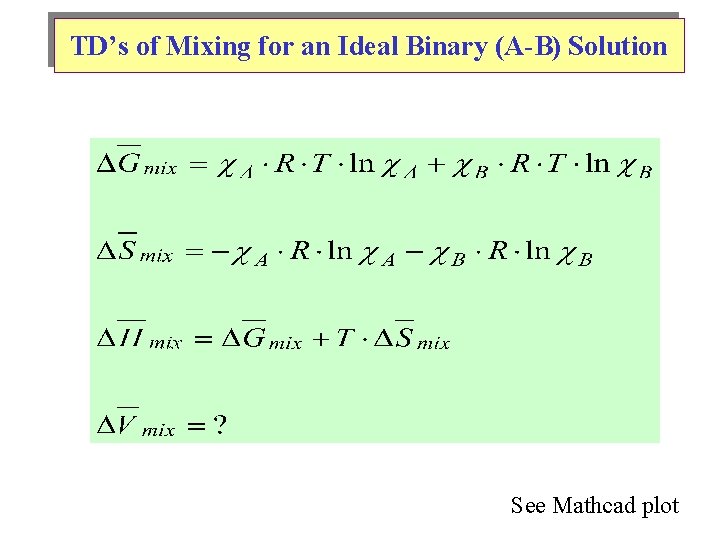
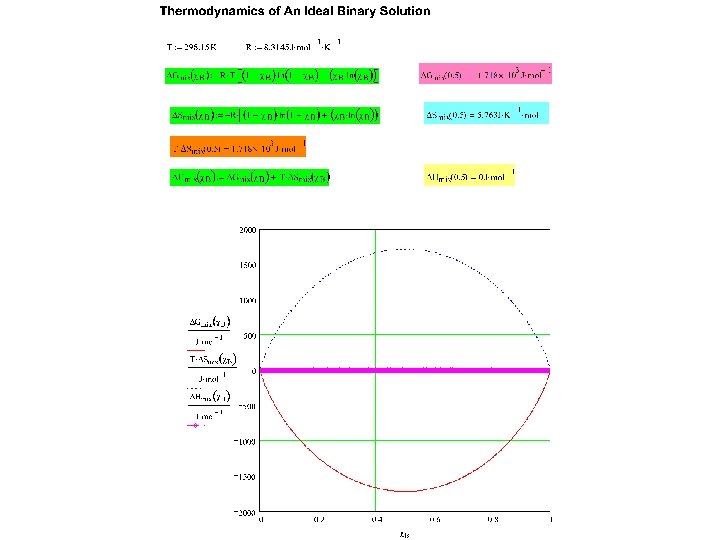
TD’s of Mixing for an Ideal Binary (A-B) Solution See Mathcad plot


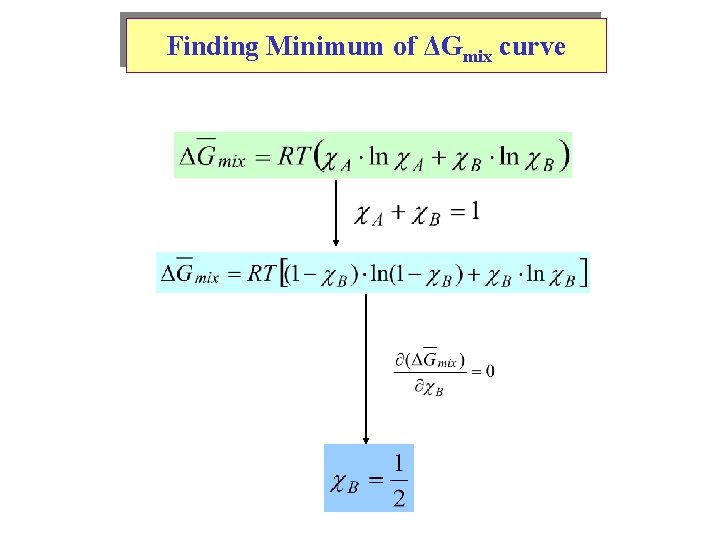
Finding Minimum of ΔGmix curve

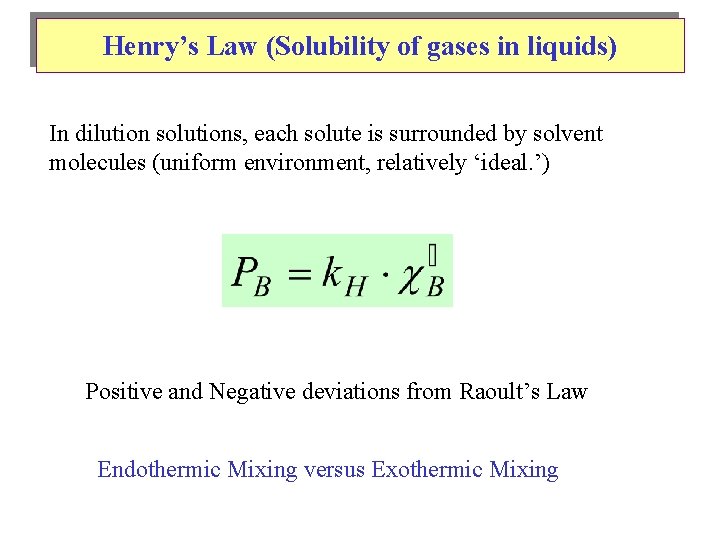
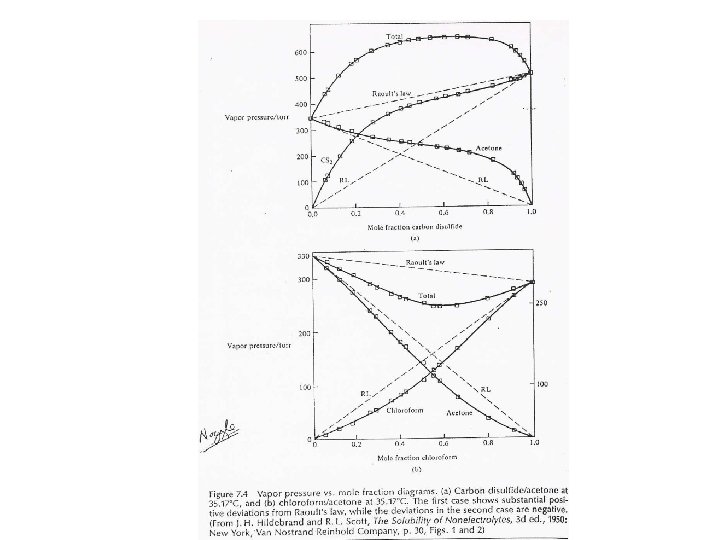
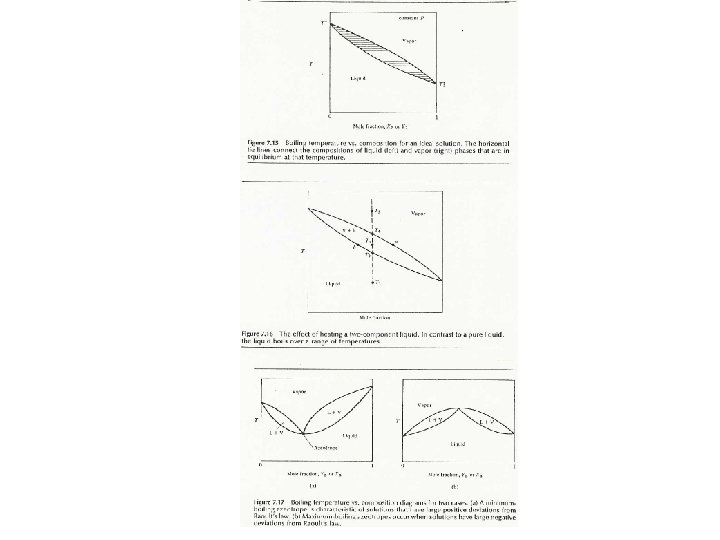
Henry’s Law (Solubility of gases in liquids) In dilution solutions, each solute is surrounded by solvent molecules (uniform environment, relatively ‘ideal. ’) Positive and Negative deviations from Raoult’s Law Endothermic Mixing versus Exothermic Mixing


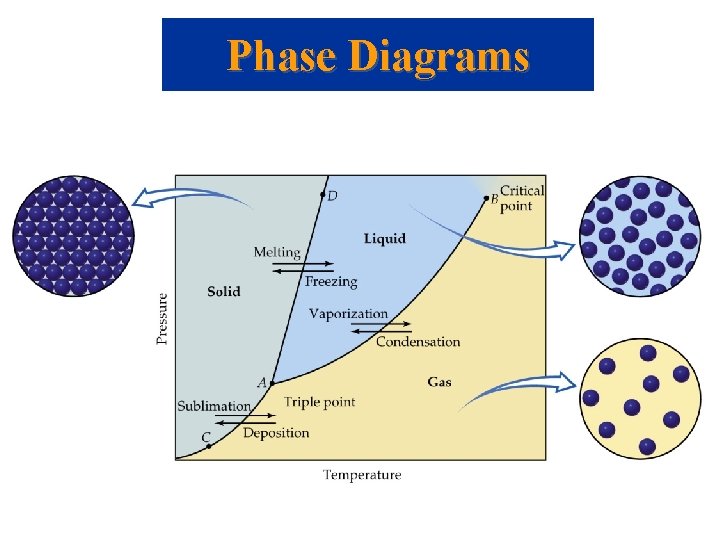
Phase Diagrams

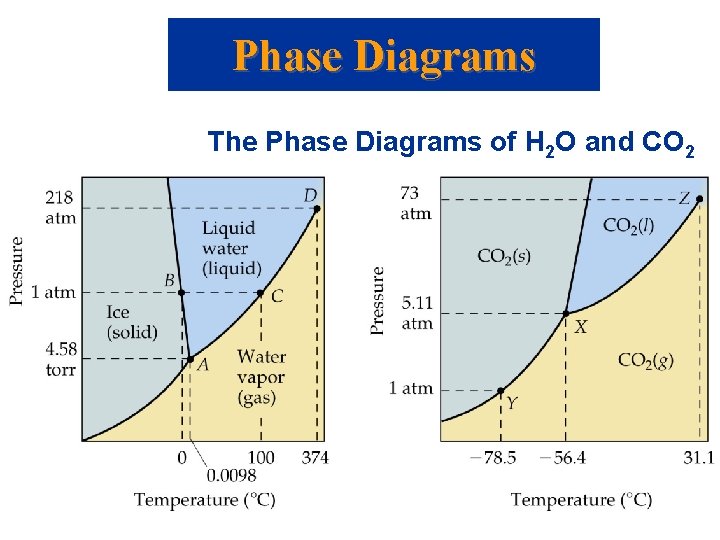
Phase Diagrams The Phase Diagrams of H 2 O and CO 2

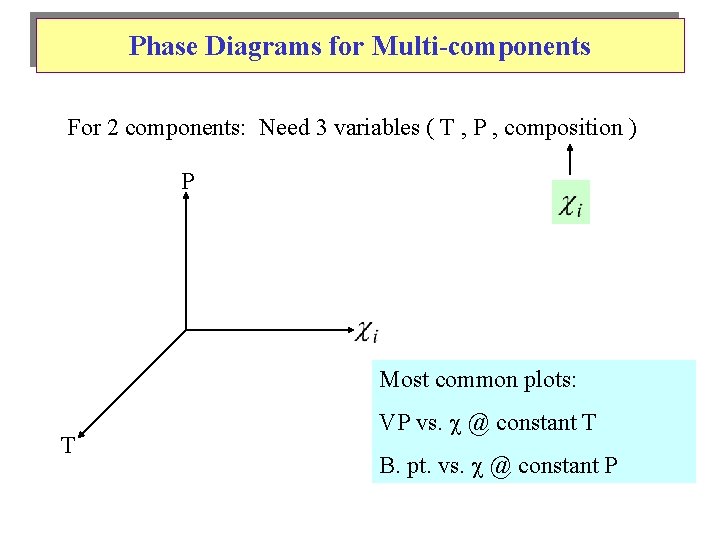
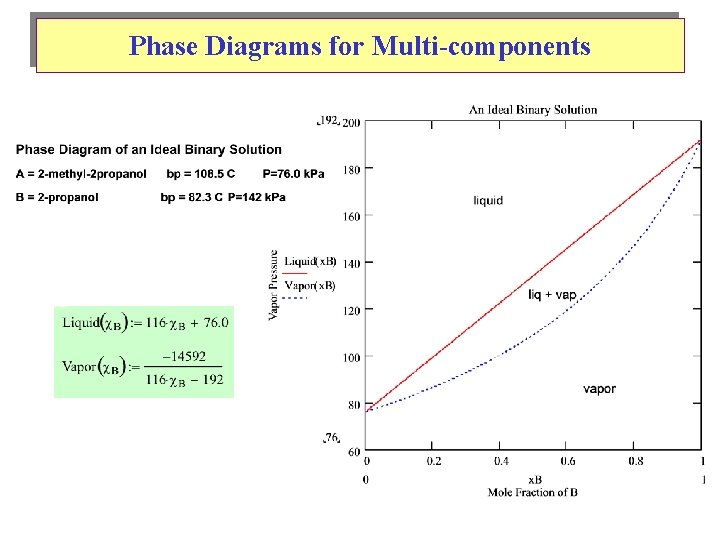
Phase Diagrams for Multi-components For 2 components: Need 3 variables ( T , P , composition ) P Most common plots: T VP vs. @ constant T B. pt. vs. @ constant P

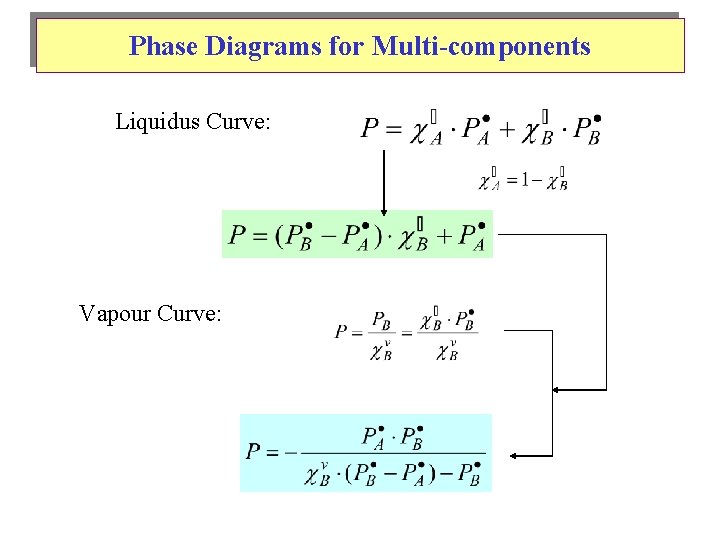
Phase Diagrams for Multi-components Liquidus Curve: Vapour Curve:

Phase Diagrams for Multi-components


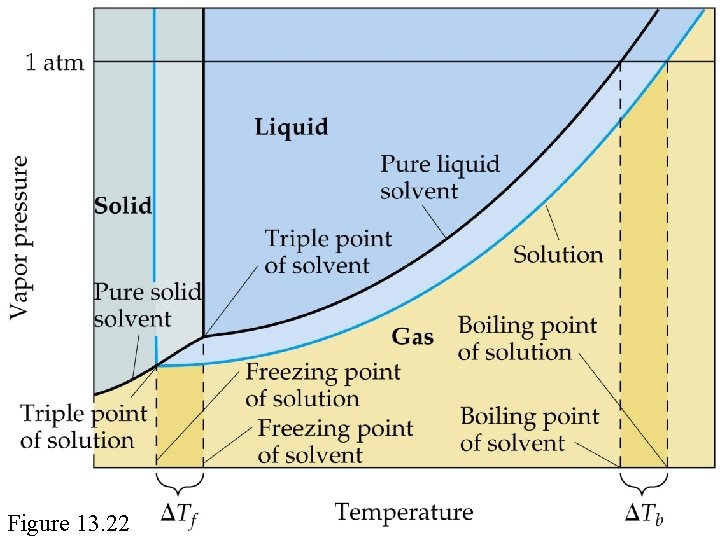
Colligative Properties Boiling-Point Elevation • Molal boiling-point-elevation constant, Kb, expresses how much Tb changes with molality, m. S: • Decrease in freezing point ( Tf) is directly proportional to molality (Kf is the molal freezing-point-depression constant):

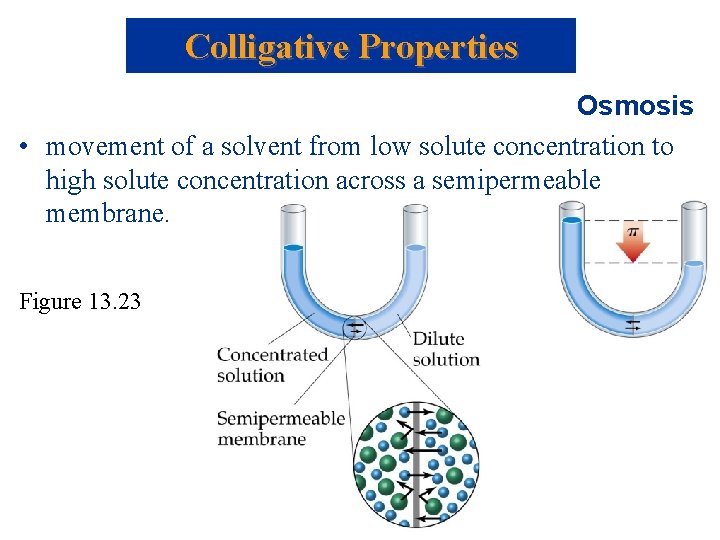
Figure 13. 22

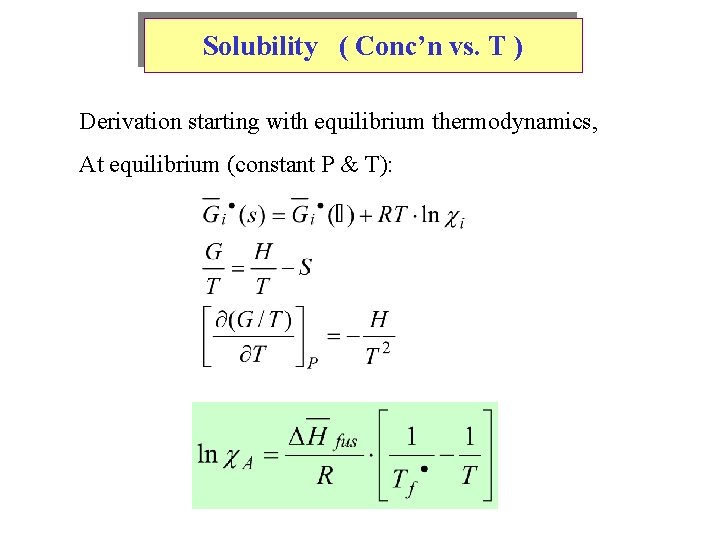
Solubility ( Conc’n vs. T ) Derivation starting with equilibrium thermodynamics, At equilibrium (constant P & T):

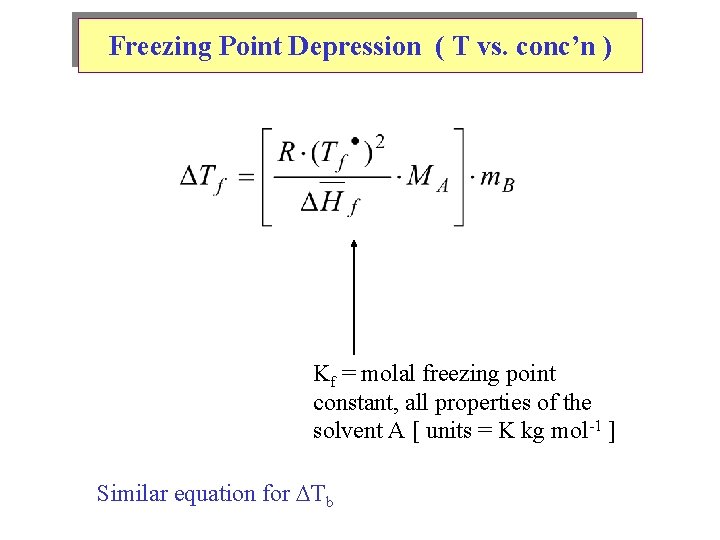
Freezing Point Depression ( T vs. conc’n ) Kf = molal freezing point constant, all properties of the solvent A [ units = K kg mol-1 ] Similar equation for Tb

Colligative Properties Osmosis • movement of a solvent from low solute concentration to high solute concentration across a semipermeable membrane. Figure 13. 23

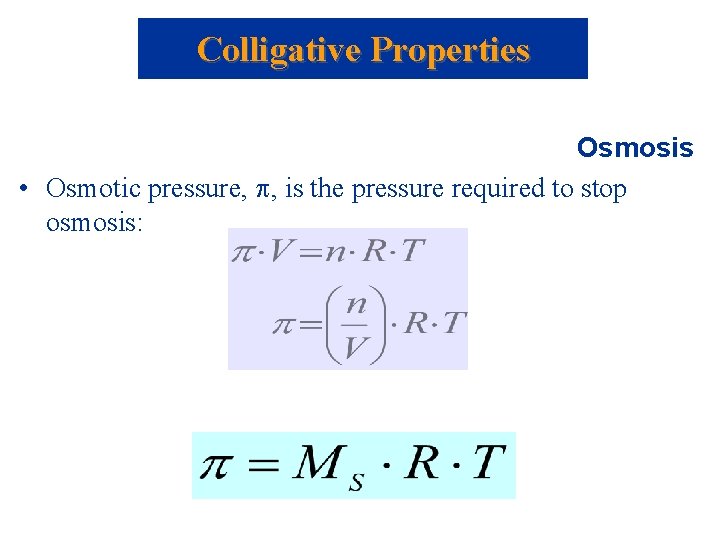
Colligative Properties Osmosis • Osmotic pressure, , is the pressure required to stop osmosis:

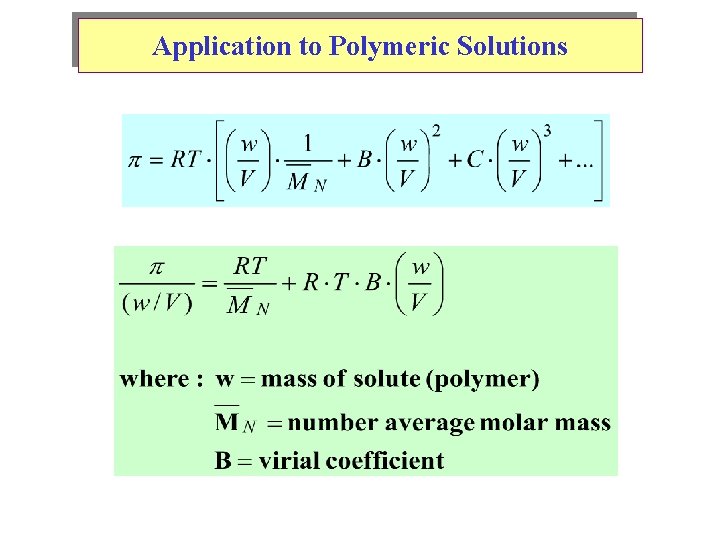
Application to Polymeric Solutions

Ideal and Dilute Solutions